Abstract
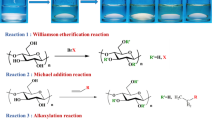
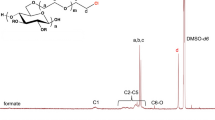
This chapter gives an overview on various possibilities of cellulose functionalization. Particular attention was paid on homogeneous-phase conversions of cellulose in reaction media based on both aprotic-dipolar solvents in combination with salts and ionic liquids. Paths for cellulose esterification via in situ activated carboxylic acids are discussed. Not only etherification and esterification of hydroxyl groups lead to novel derivatives, but also oxidation reactions, in particular catalytic approaches, are of great importance. Recent developments in the synthesis of regioselectively functionalized cellulose ethers are reviewed as well. It is demonstrated that advanced synthesis methods, in particular the application of silicon-based protecting groups, are important to get the regioselectively functionalized cellulose ethers of high structural uniformity. Moreover, novel derivatives could be prepared thereof in subsequent reactions like oxidation and 1,3-dipolar cycloaddition reaction of azide- and alkyne-containing cellulose derivatives.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abbott AP, Capper G, Davies DL, Rasheed RK (2004) Ionic liquids based upon metal halide/substituted quaternary ammonium salt. Inorg Chem 43: 3447–3452
Abbott AP, Bell TJ, Handa S, Stoddart B (2005) O-acetylation of cellulose and monosaccharides using a zinc based ionic liquid. Green Chem 7: 705–707
Arisawa M, Kato C, Kaneko H, Nishida A, Nakagawa M (2000) Concise synthesis of azacycloundecenes using ring-closing metathesis (RCM). J Chem Soc Perkin Trans 1 12:1873–1876
Bailey WF, Bobbitt JM, Wiberg KB (2007) Mechanism of the oxidation of alcohols by oxoammonium cations. J Org Chem 72:4504–4509
Banker GS, Kumar V (1995) Microfibrillated oxycellulose. US Patent 5405953
Bar-Nir BB, Kadla JF (2009) Synthesis and structural characterization of 3-O-ethylene glycol functionalized cellulose derivatives. Carbohydr Polym 76:60–67
Barthel S, Heinze T (2006) Acylation and carbanilation of cellulose in ionic liquids. Green Chem 8:301–306
Biliuta G, Fras L, Strnad S, Harabagiu V, Coseri S (2010) Oxidation of cellulose fibers mediated by nonpersistent nitroxyl radicals. J Polym Sci A Polym Chem 48:4790–4799
Borisov IM, Shirokova EN, Mudarisova RK, Muslukhov RR, Zimin YS, Medvedeva SA, Tolstikov GA, Monakov YB (2004) Kinetics of oxidation of an arabinogalactan from larch (Larix sibirica L.) in an aqueous medium in the presence of hydrogen peroxide. Russ Chem Bull 53:318–324
Brackhagen M, Heinze T, Dorn S, Koschella A (2007) Verfahren zur Herstellung aminogruppenhaltiger Cellulosederivate in ionischer Flüssigkeit. EP Patent 2072530A1
Bragd PL, Van Bekkum H, Besemer AC (2004) TEMPO-mediated oxidation of polysaccharides: survey of methods and applications. Top Catal 27:49–66
Brewster JH, Ciotti CJ Jr (1955) Dehydrations with aromatic sulfonyl halides in pyridine. A convenient method for the preparation of esters. J Am Chem Soc 77:6214–6215
Butrim SM, Bil’dyukevich TD, Butrim NS, Yurkshtovich TL (2007) Structural modification of potato starch by solutions of nitrogen (IV) oxide in CCl4. Chem Nat Compd 43:302–305
Calvini P, Conio G, Lorenzoni M, Pedemonte E (2004) Viscometric determination of dialdehyde content in periodate oxycellulose. Part I. Methodology. Cellulose 11:99–107
Calvini P, Gorassini A, Luciano G, Franceschi E (2006) FTIR and WAXS analysis of periodate oxycellulose: evidence for a cluster mechanism of oxidation. Vib Spectrosc 40:177–183
Camacho Gomez JA, Erler UW, Klemm D (1996) O,4-methoxy substituted trityl groups in 6-O protection of cellulose: homogeneous synthesis, characterization, detritylation. Macromol Chem Phys 197:953–964
Camy S, Montanari S, Rattaz A, Vignon M, Condoret JS (2009) Oxidation of cellulose in pressurized carbon dioxide. J Supercrit Fluids 51:188–196
Cao Y, Wu J, Meng T, Zhang J, He JS, Li HQ, Zhang Y (2007) Acetone-soluble cellulose acetates prepared by one-step homogeneous acetylation of cornhusk cellulose in an ionic liquid 1-allyl-3-methylimidazolium chloride (AmimCl). Carbohydr Polym 69:665–672
Cato Y, Kaminaga J, Matsuo R, Isogai A (2004) TEMPO-mediated oxidation of chitin, regenerated chitin and N-acetylated chitosan. Carbohydr Polym 58:421–426
Chang PS, Robyt JF (1996) Oxidation of primary alcohol groups of naturally occurring polysaccharides with 2,2,6,6-tetramethyl-1-piperidine oxoammonium ion. J Carbohydr Chem 15:819–830
Ciacco GT, Liebert TF, Frollini E, Heinze TJ (2003) Application of the solvent dimethyl sulfoxide/tetrabutyl-ammonium fluoride trihydrate as reaction medium for the homogeneous acylation of Sisal cellulose. Cellulose 10:125–132
Coseri S (2009) Phthalimide-N-oxyl (PINO) radical, a powerful catalytic agent: its generation and versatility towards various organic substrates. Cat Rev 51:218–292
Coseri S, Mendenhall GD, Ingold KU (2005) Mechanisms of reactions of aminoxyl (nitroxide), iminoxyl, and imidoxyl radicals with alkenes and evidence that in the presence of lead tetraacetate, N-hydroxyphthalimide reacts with alkenes by both radical and nonradical mechanisms. J Org Chem 70:4629–4636
Coseri S, Nistor G, Fras L, Strnad S, Harabagiu V, Simionescu BC (2009) Mild and selective oxidation of cellulose fibers in the presence of N-hydroxyphthalimide. Biomacromolecules 10:2294–2299
Danaee I, Jafarian M, Mirzapoor A, Gobal F, Mahjani MG (2010) Electrooxidation of methanol on NiMn alloy modified graphite electrode. Electrochim Acta 55:2093–2100
Davis NJ, Flitsch SL (1993) Selective oxidation of monosaccharide derivatives to uronic acids. Tetrahedron Lett 34:1181–1184
De Nooy AEJ, Besemer AC, Van Bekkum H (1994) Highly selective TEMPO mediated oxidation of primary alcohol groups in polysaccharides. Recl Trav Chim Pays Bas 113:165–166
De Nooy AEJ, Besemer AC, Van Beckum H (1995) Highly selective nitroxyl radical-mediated oxidation of primary alcohol groups in water-soluble glucans. Carbohydr Res 269:89–98
Deus C, Friebolin H, Siefert E (1991) Partiell acetylierte Cellulose-Synthese und Bestimmung der Substituentenverteilung mit Hilfe der 1 H NMR Spektroskopie. Makromol Chem 192:75–83
Dias GJ, Peplow PV, Teixeira F (2003) Osseous regeneration in the presence of oxidized cellulose and collagen. J Mater Sci Mater Med 14:739–745
Dorn S, Schöbitz M, Schlufter K, Heinze T (2010a) Novel cellulose products prepared by homogeneous functionalization of cellulose in ionic liquids. ACS Symp Ser 1033:275–285
Dorn S, Pfeifer A, Schlufter K, Heinze T (2010b) Synthesis of water-soluble cellulose esters applying carboxylic acid imidazolides. Polym Bull 64:845–854
Edgar KJ, Arnold KM, Blount WW, Lawniczak JE, Lowman DW (1995) Synthesis and properties of cellulose acetoacetates. Macromolecules 28:4122–4128
Edgar KJ, Pecorini TJ, Glasser WG (1998) Long-chain cellulose esters: preparation, properties, and perspective. In: Heinze T, Glasser WG (eds) Cellulose derivatives; modification, characterization and nanostructures. ACS Symposium Series 688, American Chemical Society, Washington, DC, p 38
El Seoud OA, Heinze T (2005) Organic esters of cellulose: New perspectives for old polymers. In: Heinze T (ed) Advances in polymer science: polysaccharides I, structure, characterization and use, vol 186. Springer, Heidelberg, p 103ff
El Seoud OA, Marson GA, Ciacco GT, Frollini E (2000) An efficient, one-pot acylation of cellulose under homogeneous reaction conditions. Macromol Chem Phys 201:882–889
Fenn D, Heinze T (2009) Novel 3-mono-O-hydroxyethyl cellulose: synthesis and structure characterization. Cellulose 16:853–861
Fenn D, Pfeifer A, Heinze T (2007) Studies on the synthesis of 2,6-di-O-thexyldimethylsilyl cellulose. Cell Chem Technol 41:87–91
Fenn D, Pohl M, Heinze M (2009) Novel 3-O-propargyl cellulose as a precursor for regioselective functionalization of cellulose. React Funct Polym 69:347–352
Fenselau AH, Moffatt JG (1966) Sulfoxide-carbodiimide reactions. III. Mechanism of the oxidation reaction. J Am Chem Soc 88:1762–1765
Fras L, Johansson LS, Stenius P, Laine J, Stana-Kleinschek K, Ribitsch V (2005) Analysis of the oxidation of cellulose fibres by titration and XPS. Colloid Surf A 260:101–108
Galgut PN (1990) Oxidized cellulose mesh: I. Biodegradable membrane in periodontal surgery. Biomaterials 11:561–564
Gericke M, Liebert T, Heinze T (2009a) Interaction of ionic liquids with polysaccharides – 8. Synthesis of cellulose sulfate suitable for symplex formation. Macromol Biosci 9:343–353
Gericke M, Schlufter K, Liebert T, Heinze T, Budtova T (2009b) Rheological properties of cellulose/ionic liquid solutions: from dilute to concentrated states. Biomacromolecules 10:1188–1194
Gericke M, Liebert T, Heinze T (2009c) Polyelectrolyte complex formation in ionic liquids. J Am Chem Soc 131:13220–13221
Glasser WG, Samaranayake G, Dumay M, Dave VJ (1995) Novel cellulose derivatives. III. Thermal analysis of mixed esters with butyric and hexanoic acid. J Polym Sci B Polym Phys 33:2045–2054
Glasser WG, Becker U, Todd JG (2000) Novel cellulose derivatives. VI. Preparation and thermal analysis of two novel cellulose esters with fluorine-containing substituents. Carbohydr Polym 42:393–400
Gomez-Bujedo S, Fleury E, Vignon MR (2004) Preparation of cellouronic acids and partially acetylated cellouronic acids by TEMPO/NaClO oxidation of water-soluble cellulose acetate. Biomacromolecules 5:565–571
Gräbner D, Liebert T, Heinze T (2002) Synthesis of novel adamantoyl cellulose using differently activated carbonic acid derivatives. Cellulose 9:193–201
Graenacher C (1934) Cellulose solution. US Patent 1943176
Guthrie JD (1971) Ion-exchange celluloses. In: Bikales NM, Segal L (eds) Cellulose and cellulose derivatives. Wiley-Interscience, New York
Haslam E (1980) Recent developments in methods for the esterification and protection of the carboxyl group. Tetrahedron 36:2409–2433
Hassan ML (2006) Preparation and thermal stability of new cellulose-based poly(propylene imine) and poly(amido amine) hyperbranched derivatives. J Appl Polym Sci 101:2079–2087
Hassan ML, Moorefield CN, Newkome GR (2004) Regioselective dendritic functionalization of cellulose. Macromol Rapid Commun 25:1999–2002
Hassan ML, Moorefield CN, Kotta KK, Newkome GR (2005) Regioselective combinatorial-type synthesis, characterization, and physical properties of dendronized cellulose. Polymer 46:8947–8955
Heinze T, Glasser WG (1998) The role of novel solvents and solution complexes for the preparation of highly engineered cellulose derivatives. In: Heinze T, Glasser WG (eds) Cellulose derivatives, modification, characterisation and nanostructures. ACS Symposium series (688), p 2
Heinze T, Köhler S (2010) Dimethyl sulfoxide and ammonium fluorides – novel cellulose solvents. ACS Symp Ser 1033:103–118
Heinze T, Koschella A (2008) Water-soluble 3-O-methoxyethyl cellulose: synthesis and characterization. Carbohydr Res 343:668–673
Heinze T, Schaller J (2000) New water soluble cellulose esters synthesized by an effective acylation procedure. Macromol Chem Phys 201:1214–1218
Heinze T, Röttig K, Nehls I (1994) Synthesis of 2,3-O-carboxymethylcellulose. Macromol Rapid Commun 15:311–317
Heinze U, Heinze T, Klemm D (1999) Synthesis and structure characterization of 2,3-O-carboxymethylcellulose. Macromol Chem Phys 200:896–902
Heinze T, Dicke R, Koschella A, Kull AH, Klohr EA, Koch W (2000a) Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol Chem Phys 201:627–631
Heinze T, Vieira M, Heinze U (2000b) New polymers based on cellulose. Lenzinger Ber 79:39–44
Heinze T, Liebert T, Pfeiffer K, Hussain MA (2003) Unconventional cellulose esters: synthesis, characterization, and structure property relations. Cellulose 10:283–296
Heinze T, Schwikal K, Barthel S (2005) Ionic liquids as reaction medium in cellulose functionalization. Macromol Biosci 5:520–525
Heinze T, Koschella A, Brackhagen M, Engelhardt J, Nachtkamp K (2006) Studies on non-natural deoxyammonium cellulose. Macromol Symp 244:74–82
Heinze T, Pohl M, Schaller M, Meister F (2007) Novel bulky esters of cellulose. Macromol Biosci 7:1225–1231
Heinze T, Schöbitz M, Pohl M, Meister F (2008a) Interactions of ionic liquids with polysaccharides: IV. Dendronization of 6-azido-6-deoxy cellulose. J Polym Sci A Polym Chem 46:3853–3859
Heinze T, Pfeifer A, Petzold K (2008b) Regioselective reaction of cellulose with tert-butyldimethylsilyl chloride in N,N-dimethyl acetamide/LiCl. BioResources 3:79–90
Heinze T, Pfeifer A, Sarbova V, Koschella A (2011) 3-O-Propyl cellulose: cellulose ether with exceptionally low flocculation temperature. Polym Bull 66:1219–1229
Hirota M, Tamura N, Saito T, Isogai A (2009a) Oxidation of regenerated cellulose with NaClO2 catalyzed by TEMPO and NaClO under acid-neutral conditions. Carbohydr Polym 78:330–335
Hirota M, Tamura N, Saito T, Isogai A (2009b) Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 16:841–851
Hirrien M, Chevillard C, Desbrieres J, Axelos MAV, Rinaudo M (1998) Thermogelation of methylcelluloses: new evidence for understanding the gelation mechanism. Polymer 39:6251–6259
Hon DN, Yan HJ (2001) Cellulose furoate. I. Synthesis in homogeneous and heterogeneous systems. J Appl Polym Sci 81:2649–2655
Huang K, Xia J, Li M, Lian J, Yang X, Lin G (2011) Homogeneous synthesis of cellulose stearates with different degrees of substitution in ionic liquid 1-butyl-3-methylimidazolium chloride. Carbohydr Polym 83:1631–1635
Husemann E, Siefert E (1969) N-äthyl-pyridinium-chlorid als Lösungsmittel und Reaktionsmedium für Cellulose. Makromol Chem 128:288–291
Hussain MA, Liebert T, Heinze T (2004a) Acylation of cellulose with N,N’ -carbonyldiimidazole-activated acids in the novel solvent dimethyl sulfoxide/tetrabutylammonium fluoride. Macromol Rapid Commun 25:916–920
Hussain MA, Liebert T, Heinze T (2004b) First report on a new esterification method for cellulose. Polym News 29:14–17
Ibrahim AA, Nada AMA, Hagemann U, El Seoud OA (1996) Preparation of dissolving pulp from sugar cane bagasse, and its acetylation under homogeneous solution condition. Holzforschung 50:221–225
Illy N (2006) Diploma Thesis, Friedrich Schiller University of Jena
Isogai A, Kato Y (1998) Preparation of polyuronic acid from cellulose by TEMPO-mediated oxidation. Cellulose 5:153–164
Isogai T, Saito T, Isogai A (2010) TEMPO electromediated of some polysaccharides including regenerated cellulose fiber. Biomacromolecules 11:1593–1599
Itagaki H, Tokai M, Kondo T (1997) Physical gelation process for cellulose whose hydroxyl groups are regioselectively substituted by fluorescent groups. Polymer 38:4201–4205
Iwata T, Azuma J, Okamura K, Muramoto M, Chun B (1992) Preparation and n.m.r. assignments of cellulose mixed esters regioselectively substituted by acetyl and propanoyl groups. Carbohydr Res 224:277–283
Jin B, Wu W (2005) Compositions for veterinary and medical applications. WO Patent 2005020997
Johansson EE, Lind J (2005) Free radical mediated cellulose degradation during high consistency ozonation. J Wood Chem Technol 25:171–186
Just EK, Majewicz TG (1985) Cellulose ethers. In: Mark HF, Bikales NM, Overberger CG, Menges G, Kroschwitz JI (eds) Encyclopedia of polymer science and engineering (2nd ed). Wiley, New York, p III/226ff
Kadla JF, Asfour FH, Bar-Nir B (2007) Micropatterned thin film honeycomb materials from regiospecifically modified cellulose. Biomacromolecules 8:161–165
Kamitakahara H, Koschella A, Mikawa Y, Nakatsubo F, Heinze T, Klemm D (2008) Syntheses and comparison of 2,6-Di-O-methyl celluloses from natural and synthetic celluloses. Macromol Biosci 8:690–700
Kato T, Miyagawa A, Carmelita M, Kasuya Z, Hatanaka K (2009) Development of membrane filter with oligosaccharide immobilized by click chemistry for influenza virus adsorption. Open Chem Biomed Methods J 2:13–17
Katritzky AR, Zhang Y, Singh SK (2003) Efficient conversion of carboxylic acids into N-acylbenzotriazoles. Synthesis 18:2795–2798
Katritzky AR, Cai C, Singh SK (2006) Efficient microwave access to polysubstituted amidines from imidoylbenzotriazoles. J Org Chem 71:3375–3380
Kern H, Choi S, Wenz G, Heinrich J, Erhardt L, Mischnik P, Garidel P, Blume A (2000) Synthesis, control of substitution pattern and phase transitions of 2,3-di-O-methylcellulose. Carbohydr Res 326:67–79
Kitaoka T, Isogai A, Onabe F (1999) Chemical modification of pulp fibers by TEMPO-mediated oxidation. Nord Pulp Pap Res J 14:279–284
Klemm D, Stein A (1995) Silylated cellulose materials in design of supramolecular structures of ultrathin cellulose films. J Macromol Sci A Pure Appl Chem A32:899–904
Klemm D, Schnabelrauch M, Stein A, Philipp B, Wagenknecht W, Nehls I (1990) Recent results from homogeneous esterification of cellulose using soluble intermediate compounds. Das Papier 44:624–632
Klemm D, Philip B, Heinze T, Heinze U, Wagenknecht W (1998) Cellulose sulfates. In: Comprehensive cellulose chemistry, vol 2, Wiley VCH, New York, pp 115–133
Klohr EA, Koch W, Klemm D, Dicke R (2000) Regioselektiv substituierte Ester von Oligo- und Polysacchariden und Verfahren zu ihrer Herstellung. DE Patent 19951734, CAN Patent 133:224521
Köhler S, Heinze T (2007) Efficient synthesis of cellulose furoates in 1-N-butyl-3-methylimidazolium chloride. Cellulose 14:489–495
Köhler S, Liebert T, Schöbitz M, Schaller J, Meister F, Günther W, Heinze T (2007) Interactions of ionic liquids with polysaccharides - 1: unexpected acetylation of cellulose with 1-ethyl-3-methylimidazolium acetate. Macromol Rapid Commun 28:2311–2317
Kolb HC, Finn MG, Sharpless KB (2001) Click-Chemie: diverse chemische Funktionalität mit einer Handvoll guter Reaktionen. Angew Chem 113:2056–2075
Kondo T (1993) Preparation of 6-O-alkylcelluloses. Carbohydr Res 238:231–240
Kondo T (1997) The relationship between intramolecular hydrogen bonds and certain physical properties of regioselectively substituted cellulose derivatives. J Polym Sci B Polym Phys 35:717–723
Kondo T, Gray DG (1991) The preparation of O-methyl- and O-ethyl-celluloses having controlled distribution of substituents. Carbohydr Res 220:173–183
Kondo T, Sawatari C, Manley R, St J, Gray DG (1994) Characterization of hydrogen bonding in cellulose-synthetic polymer blend systems with regioselectively substituted methylcellulose. Macromolecules 27:210–215
Kondo T, Koschella A, Heublein B, Klemm D, Heinze T (2008) Hydrogen bond formation in regioselectively functionalized 3-mono-O-methyl cellulose. Carbohydr Res 343:2600–2604
Koschella A, Klemm D (1997) Silylation of cellulose regiocontrolled by bulky reagents and dispersity in the reaction media. Macromol Symp 120:115–125
Koschella A, Haucke G, Heinze T (1997) New fluorescence active cellulosics prepared by a convenient acylation reaction. Polym Bull 39:597–604
Koschella A, Heinze T, Klemm D (2001) First synthesis of 3-O-functionalized cellulose ethers via 2,6-di-O-protected silyl cellulose. Macromol Biosci 1:49–54
Koschella A, Fenn D, Heinze T (2006) Water soluble 3-mono-O-ethyl cellulose: synthesis and characterization. Polym Bull 57:33–41
Koschella A, Richter M, Heinze T (2010) Novel cellulose-based polyelectrolytes synthesized via click reaction. Carbohydr Res 345:1028–1033
Kostic M, Potthast A, Rosenau T, Kosma P, Sixta H (2006) A novel approach to determination of carbonyl groups in DMAc/LiCl-insoluble pulps by fluorescence labeling. Cellulose 13:429–435
Liebert T, Heinze T (2005) Tailored cellulose esters: synthesis and structure determination. Biomacromolecules 6:333–340
Liebert T, Hänsch C, Heinze T (2006) Click chemistry with polysaccharides. Macromol Rapid Commun 27:208–213
Liu H-Q, Zhang L-N, Takaragi A, Miyamoto T (1997) Water solubility of regioselectively 2,3-O-substituted carboxymethylcellulose. Macromol Rapid Commun 18:921–925
Liu CF, Sun RC, Zhang AP, Ren JL, Wang XA, Geng ZC (2006) Structural and thermal characterization of sugarcane bagasse cellulose succinates prepared in ionic liquid. Polym Degrad Stabil 91:3040–3047
Liu CF, Sun RC, Zhang AP, Qin M-H, Ren J-L, Wang X-A (2007a) Preparation and characterization of phthalated cellulose derivatives in room-temperature ionic liquid without catalysts. Agricult Food Chem 55:2399–2406
Liu CF, Sun RC, Zhang AP, Ren JL, Wang XA, Qin MH, Chaod ZN, Luod W (2007b) Homogeneous modification of sugarcane bagasse cellulose with succinic anhydride using a ionic liquid as reaction medium. Carbohydr Res 342:919–926
Manhas MS, Mohammed F, Khan Z (2007) A kinetic study of oxidation of β-cyclodextrin by permanganate in aqueous media. Colloid Surf 295:165–171
McCormick CL, Callais PA (1987) Derivatization of cellulose in lithium chloride and N-N-dimethylacetamide solutions. Polymer 28:2317–2323
McCormick CL, Lichatowich DK (1979) Homogeneous solution reactions of cellulose, chitin, and other polysaccharides to produce controlled-activity pesticide systems. J Polym Sci C Polym Lett 17:479–484
Nagel MCV, Heinze T (2010) Esterification of cellulose with acyl-1 H-benzotriazole. Polym Bull 65:873–881
Nojiri M, Kondo T (1996) Application of regioselectively substituted methylcelluloses to characterize the reaction mechanism of cellulase. Macromolecules 29:2392–2395
Östmark E, Lindqvist J, Nyström D, Malmström E (2007) Dendronized hydroxypropyl cellulose – synthesis and characterization of biobased nanoobjects. Biomacromolecules 8:3815–3822
Painter TJ (1977) Preparation and periodate oxidation of C-6-oxycellulose: conformational interpretation of hemiacetal stability. Carbohydr Res 55:95–103
Pameijer CH, Jensen S (2007) Agents and devices for providing blood clotting functions to wounds. US Patent 20070190110
Pawlowski WP, Sankar SS, Gilbert RD, Fornes RD (1987) Synthesis and solid state 13C-NMR studies of some cellulose derivatives. J Polym Sci A Polym Chem 25:3355–3362
Petzold K, Klemm D, Heublein B, Burchard W, Savin G (2004) Investigations on structure of regioselectively functionalized celluloses in solution exemplified by using 3-O-alkyl ethers and light scattering. Cellulose 11:177–193
Philipp B, Wagenknecht W, Wagenknecht M, Nehls I, Klemm D, Stein A, Heinze T, Heinze U, Helbig K (1995) Regioselective esterification and etherification of cellulose and cellulose derivatives. 1. Problems and description of the reaction systems. Das Papier 49:3–7
Pohl M, Heinze T (2008) Novel biopolymer structures synthesized by dendronization of 6-deoxy-6-aminopropargyl cellulose. Macromol Rapid Commun 29:1739–1745
Pohl M, Schaller J, Meister F, Heinze T (2008a) Novel bulky esters of biopolymers: dendritic cellulose. Macromol Symp 262:119–128
Pohl M, Schaller J, Meister F, Heinze T (2008b) Selectively dendronized cellulose: synthesis and characterization. Macromol Rapid Commun 29:142–148
Pohl M, Morris GA, Harding SE, Heinze T (2009a) Studies on the molecular flexibility of novel dendronized carboxymethyl cellulose derivatives. Eur Polym J 45:1098–1110
Pohl M, Michaelis N, Meister F, Heinze T (2009b) Biofunctional surfaces based on dendronized cellulose. Biomacromolecules 10:382–389
Potthast A, Rohrling J, Rosenau T, Borgards A, Sixta H, Kosma P (2003) A novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. 3 Monitoring oxidative processes. Biomacromolecules 4:743–749
Regiani AM, Frollini E, Marson GA, Arantes GM, El Seoud OA (1999) Some aspects of acylation of cellulose under homogeneous solution conditions. J Polym Sci A Polym Chem 37:1357–1363
Ringot C, Sol V, Granet R, Krausz P (2009) Porphyrin-grafted cellulose fabric: new photobactericidal material obtained by “Click-Chemistry” reaction. Mater Lett 63:1889–1891
Saito T, Isogai A (2004) TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 5:1983–1989
Samaranayake G, Glasser WG (1993) Cellulose derivatives with low DS. I. A novel acylation system. Carbohydr Polym 22:1–7
Schaller J, Heinze T (2005) Studies on the synthesis of 2,3-O-hydroxyalkyl ethers of cellulose. Macromol Biosci 5:58–63
Schlufter K, Schmauder HP, Dorn S, Heinze T (2006) Efficient homogeneous chemical modification of bacterial cellulose in the ionic liquid 1-N-butyl-3-methylimidazolium chloride. Macromol Rapid Commun 27:1670–1676
Schumann K, Pfeifer A, Heinze T (2009) Novel cellulose ethers: synthesis and structure characterization of 3-mono-o-(3’ -hydroxypropyl) cellulose. Macromol Symp 280:86–94
Schweiger RG (1972) Polysaccharide sulfates. I. Cellulose sulfate with a high degree of substitution. Carbohydr Res 21:219–228
Sealey JE, Samaranayake G, Todd JG, Glasser WG (1996) Novel cellulose derivatives. IV. Preparation and thermal analysis of waxy esters of cellulose. J Polym Sci B Polym Phys 34:1613–1620
Sealey JE, Frazier CE, Samaranayake G, Glasser WG (2000) Novel cellulose derivatives. V. Synthesis and thermal properties of esters with trifluoroethoxy acetic acid. J Polym Sci B Polym Phys 38:486–494
Sharma JB, Malhotra M (2003) Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynecol Obstet 82:221–222
Sharma JB, Malhotra M, Pundir P (2003) Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynecol Obstet 83:271–275
Shimizu Y, Hayashi J (1988) A new method for cellulose acetylation with acetic acid. Sen-i Gakkaishi 44:451–456
Shimizu Y, Nakayama A, Hayashi J (1991) Acetylation of cellulose with carboxylate salts. Cell Chem Technol 25:275–281
Staab HA (1962) Neuere Methoden der präperativen organischen Chemie IV Synthesen mit heterocyclischen Amiden (Azoliden). Angew Chem 74:407–423
Stadler PA (1978) Eine einfache Veresterungsmethode im Eintopf-Verfahren. Helv Chim Acta 61:1675–1681
Sun H, DiMagno SG (2005) Anhydrous tetrabutylammonium fluoride. J Am Chem Soc 127:2050–2051
Sun S, Foster TJ, MacNaughtan W, Mitchell JR, Fenn D, Koschella A, Heinze T (2009) Self-association of cellulose ethers with random and regioselective distribution of substitution. J Polym Sci B Polym Phys 47:1743–1752
Swatloski RP, Spear SK, Holbrey JD, Rogers RD (2002) Dissolution of cellulose with ionic liquids. J Am Chem Soc 124:4974–4975
Tahiri C, Vignon MR (2000) TEMPO-oxidation of cellulose: synthesis and characterisation of polyglucuronans. Cellulose 7:177–188
Takaragi A, Minoda M, Miyamoto T, Liu HQ, Zhang LN (1999) Reaction characteristics of cellulose in the LiCl/1,3-dimethyl-2-imidazolidinone solvent system. Cellulose 6:93–102
Terbojevich M, Cosani A, Focher B, Gastaldi G, Wu W, Marsano E, Conio G (1999) Solution properties and mesophase formation of 4-phenyl-benzoylcellulose. Cellulose 6:71–87
Wagenknecht W (1996) Regioselectively substituted cellulose derivatives by modification of commercial cellulose acetates. Das Papier 50:712–720
Wagenknecht W, Phillip B, Keck M (1985) Zur Acylierung von Cellulose nach Auflösung in O-basischen Lösemittelsysteme. Acta Polym 36:697–698
Wang Z-M, Li L, Xiao K-J, Wu J-Y (2009) Homogeneous sulfation of bagasse cellulose in an ionic liquid and anticoagulation activity. Bioresour Technol 4:1687–1690
Williamson SL, McCormick CL (1998) Cellulose derivatives synthesized via isocyanate and activated ester pathways in homogeneous solutions of lithium chloride N,N-dimethylacetamide. J Macromol Sci A Pure Appl Chem A35:1915–1927
Wiseman DM, Saferstein L, Wolf S (2002) Bioresorbable oxidized cellulose composite material for prevention of postsurgical adhesions. US Patent 6500777
Wu J, Zhang J, Zhang H, He J, Ren Q, Guo M (2004) Homogeneous acetylation of cellulose in a new ionic liquid. Biomacromolecules 5:266–268
Yoshida Y, Yanagisawa M, Isogai A, Suguri N, Sumikawa N (2005) Preparation of polymer brush-type cellulose β-ketoesters using LiCl/1,3-dimethyl-2-imidazolidinone as a solvent. Polymer 46:2548–2557
Zhang C, Daly WH (2005) Synthesis and characterization of a trifunctional amidoamine cellulose derivative. Polymer Prepr 46:707–710
Zhang ZB, McCormick CL (1997) Structopendant unsaturated cellulose esters via acylation in homogeneous lithium chloride/N, N-dimethylacetamide solutions. J Appl Polym Sci 66:293–305
Zhang H, Wu J, Zhang J, He JS (2005) 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 38:8272–8277
Zhang C, Price LM, Daly HD (2006) Synthesis and Characterization of a trifunctional amidoamine cellulose derivative. Biomacromolecules 7:139–145
Zhang J, Wu J, Cao Y, Sang S, Zhang J, He J (2009a) Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 16:299–308
Zhang Y, Zhang L, Shuang S, Feng F, Qiao J, Guo Y, Choi MMF, Dong C (2010) Electro-oxidation of methane on roughened palladium electrode in acidic electrolytes at ambient temperatures. Anal Lett 43:1055–1065
Zhao GL, Hafren J, Deiana L, Cordova A (2010) Heterogeneous “Organoclick” Derivatization of Polysaccharides: Photochemical Thiol-ene Click Modification of Solid Cellulose. Macromol Rapid Commun 31:740–744
Zimnitski DS, Yurkshtovich TL, Bychkovsky PM (2004) Synthesis and characterization of oxidized cellulose. J Polym Sci A Polym Chem 42:4785–4791
Acknowledgments
We are indebted to Marcel Meiland, Marc Kostag, Jana Wotschadlo, and Torsten Jordan for their technical assistance. One of the authors (S. Coseri) acknowledges the financial support of European Social Fund—“Cristofor I. Simionescu” Postdoctoral Fellowship Programme (ID POSDRU/89/1.5/S/55216), Sectoral Operational Programme Human Resources Development 2007–2013.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag/WIen
About this chapter
Cite this chapter
Heinze, T., Koschella, A., Liebert, T., Harabagiu, V., Coseri, S. (2012). Cellulose: Chemistry of Cellulose Derivatization. In: Navard, P. (eds) The European Polysaccharide Network of Excellence (EPNOE). Springer, Vienna. https://doi.org/10.1007/978-3-7091-0421-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0421-7_10
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0420-0
Online ISBN: 978-3-7091-0421-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)