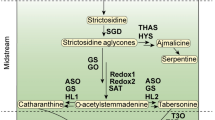
Abstract
Catharanthus roseus is an ornamental plant belonging to the Apocynaceae family which produces flowers of different color for most of the year. Besides its importance as an ornamental plant, its interest today is centered on its capacity to biosynthesize a great variety of terpenoid indole alkaloids (TIAs), which have a high added value due to their wide spectrum of pharmaceutical applications. The most important TIAs are the two antitumoral alkaloids, vinblastine and vincristine. Likewise, C. roseus also produces ajmalicine used as antihypertensive and serpentine used as sedative. The high cost of these alkaloids is due to the very small amounts that occur in C. roseus and the difficulty of their extraction which is carried out in the presence of many other compounds. This problem has created the need to find alternative sources to produce these compounds. In this respect, plant tissue/cell cultures could be a useful alternative source of pharmacologically active C. roseus alkaloids, but, even so, these have only been obtained in very low concentrations and after a substantial amount of research. This problem has stimulated intense research into the biosynthesis of TIAs and in the regulation of its pathways, with the aim of increasing the production of these high-value compounds by biotechnological approaches.
The aim of this chapter is centered on different strategies to improve TIA production which have been developed, including screening and selection of high-yield cell lines, optimization of culture conditions, feeding and elicitation strategies, and the metabolic engineering of TIA biosynthetic pathway. An up-to-date view on the biosynthesis of TIAS is also given. Although not yet successful, metabolic engineering offers the most promising perspective for improving TIA production in the future, as increases the knowledge of the genetic determination and regulation of the TIA pathway.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- 16OMT:
-
16-hydroxytabersonine 16-O-methyltransferase
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- AVLBS:
-
Anhydrovinblastine synthase
- BA:
-
Benzyladenine
- CR:
-
Cathenamine reductase
- CS:
-
Cathenamine synthase
- D4H:
-
Desacetoxyvindoline-4-hydroxylase
- DAT:
-
Deacetylvindoline-4-O-acetyltransferase
- DMAPP:
-
Dimethylallyl diphosphate
- DW:
-
Dry weight
- FW:
-
Fresh weight
- G10H:
-
Geraniol-10-hydroxylase
- GD:
-
Geissoschizine dehydrogenase
- GPP:
-
Geranyl diphosphate
- GPP synthase:
-
Geranyl diphosphate synthase
- IAA:
-
Indole-3-acetic acid
- IPP isomerase:
-
Isopentenyl-diphosphate isomerase
- IPP:
-
Isopentenyl diphosphate
- LAMT:
-
Loganic acid methyltransferase
- MeJa:
-
Methyl jasmonate
- MEP:
-
2-methyl-erythritol 4-phosphate
- NMT:
-
N-methyltransferase
- PNAE:
-
Polyneuridine aldehyde esterase
- Prx:
-
Peroxidases
- SBE:
-
Sarpagan bridge enzyme
- SGD:
-
Strictosidine β-glucosidase
- SLS:
-
Secologanin synthase
- STR:
-
Strictosidine synthase
- T16H:
-
Tabersonine 16-hydroxylase
- TDC:
-
Tryptophan decarboxylase
- TIAs:
-
Terpenoid indole alkaloids
- VH:
-
Vinorine hydroxylase
- VS:
-
Vinorine synthase
References
van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:1241–1253.
Loyola-Vargas VM, Galaz-Ávalos RM, Kú-Cauich R (2007) Catharanthus biosynthetic enzymes: the road ahead. Phytochem Rev 6:307–339. doi:10.1007/s11101-007-9064-2
Guirimand G, Guihur A, Poutrain P, Hericourt F, Mahroug S, St-Pierre B, Burlat V, Courdavault V (2011) Spatial organization of the vindoline biosynthetic pathway in Catharanthus roseus. J Plant Physiol 168:549–557. doi:10.1016/j.jplph.2010.08.01
Mahroug S, Burlat V, St-Pierre B (2007) Cellular and sub-cellular organisation of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev 6:363–381. doi:10.1007/s11101-006-9017-1
Memelink J, Gantet P (2007) Transcription factors involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Phytochem Rev 6:353–362. doi:10.1007/s11101-006-9051-z
Shukla AK, Shasany AK, Gupta MM, Khanuja SPS (2006) Transcriptome analysis in Catharanthus roseus leaves and roots for comparative terpenoid indole alkaloid profiles. J Exp Bot 57:3921–3932. doi:10.1093/jxb/erl146
Rischer H, Orešič M, Seppänen-Laakso T, Katajamaa M, Lammertyn F, Ardiles-Diaz W, Van Montagu MCE, Inzé D, Oksman-Caldentey KM, Goossens A (2006) Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc Natl Acad Sci USA 103:5614–5619. doi:10.1073/pnas.0601027103
Contin A, van der Heijden R, Lefeber AW, Verpoorte R (1998) The iridoid glucoside secologanin is derived from the novel triose phosphate/pyruvate pathway in a Catharanthus roseus cell culture. FEBS Lett 434:413–416. doi:10.1016/S0014-5793(98)01022-9
Ziegler J, Facchini PJ (2008) Alkaloid biosynthesis: metabolism and trafficking. Annu Rev Plant Biol 59:735–769. doi:10.1146/annurev.arplant.59.032607.092730
Collu G, Unver N, Peltenburg-Looman AMG, van der Heijden R, Verpoorte R, Memelink J (2001) Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett 508:215–220. doi:10.1016/S0014-5793(01)03045-9
Fernández JA, Kurz WGW, De Luca V (1989) Conformation dependent inactivation of tryptophan decarboxylase from Catharanthus roseus. Biochem Cell Biol 67:730–734. doi:10.1139/o89-109
Islas-Flores IR, Loyola-Vargas VM, Miranda-Ham ML (1994) Tryptophan decarboxylase activity in transformed roots from Catharanthus roseus and its relationship to tryptamine, ajmalicine, and catharanthine accumulation during the culture cycle. In Vitro Cell Dev Biol Plant 30:81–83. doi:10.1007/BF02632125
Pasquali G, Goddijn OJM, De Waal A, Verpoorte R, Schilperoort RA, Hoge JHC, Memelink J (1992) Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol Biol 18:1121–1131. doi:10.1007/BF00047715
Goddijn OJM, de Kam RJ, Zanetti A, Schilperoort RA, Hoge JHC (1992) Auxin rapidly down-regulates transcription of the tryptophan decarboxylase gene from Catharanthus roseus. Plant Mol Biol 18:1113–1120. doi:10.1007/BF00047714
Ouwerkerk PBF, Hallard D, Memelink J (1999) Identification of UV-B light responsive regions in the promoter of the tryptophan decarboxylase gene from Catharanthus roseus. Plant Mol Biol 41:491–503. doi:10.1023/A:1006321100550
El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6:277–305. doi:10.1007/s11101-006-9047-8
Canel C, Lopes-Cardoso MI, Whitmer S, Van Der Fits L, Pasquali G, van der Heijden R, Hoge JHC, Verpoorte R (1998) Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419. doi:10.1007/s004250050338
Ruppert M, Ma XY, Stockigt J (2005) Alkaloid biosynthesis in Rauvolfia – cDNA cloning of major enzymes of the ajmaline pathway. Curr Org Chem 9:1431–1444. doi:10.2174/138527205774370540
Pasquali G, Porto DD, Fett-Neto AG (2006) Metabolic engineering of cell cultures versus whole plant complexity in production of bioactive monoterpene indole alkaloids: recent progress related to old dilemma. J Biosci Bioeng 101:287–296. doi:10.1263/jbb.101.287
Kutchan TM (1993) Strictosidine: from alkaloid to enzyme to gene. Phytochemistry 32:493–506. doi:10.1016/S0031-9422(00)95128-8
Geerlings A, Martínez-Lozano Ibañez M, Memelink J, van der Heijden R, Verpoorte R (2000) Molecular cloning and analysis of strictosidine β-d-glucosidase, an enzyme in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Biol Chem 275:3051–3056. doi:10.1074/jbc.275.5.3051
von Schumann G, Gao S, Stöckigt J (2002) Vomilenine reductase–a novel enzyme catalyzing a crucial step in the biosynthesis of the therapeutically applied antiarrhythmic alkaloid ajmaline. Bioorg Med Chem 10:1913–1918. doi:10.1016/S0968-0896(01)00435-7
Falkenhagen H, Stöckigt J (1995) Enzymatic biosynthesis of vomilenine, a key intermediate of the ajmaline pathway, catalyzed by a novel cytochrome P450-dependent enzyme from plant cell cultures of Rauwolfia serpentina. Z Naturforsch 50:45–53. doi:35400005971172.0070
Meijer AH, Verpoorte R, Hoge JHC (1993) Regulation of enzymes and genes involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Plant Res 3:145–164. doi:35400002408251.0130
Blom TJM, Sierra M, van Vliet TB, Franke-van Dijk MEI, Koning P, van Iren F, Verpoorte R, Libbenga KR (1991) Uptake and accumulation of ajmalicine into isolated vacuoles of cultured cells of Catharanthus roseus (L.) G. Don. and its conversion into serpentine. Planta 183:170–177. doi:10.1007/BF00197785
Schröder G, Unterbusch E, Kaltenbach M, Schmidt J, Strack D, De Luca V, Schröder J (1999) Light-induced cytochrome P450-dependent enzyme in indole alkaloid biosynthesis: tabersonine 16-hydroxylase. FEBS Lett 458:97–102. doi:10.1016/S0014-5793(99)01138-2
Levac D, Murata J, Kim WS, De Luca V (2008) Application of carborundum abrasion for investigating the leaf epidermis: molecular cloning of Catharanthus roseus 16-hydroxytabersonine-16-O-methyltransferase. Plant J 53:225–236. doi:10.1111/j.1365-313X.2007.03337.x
De Luca V, Cutler AJ (1987) Subcellular localization of enzymes involved in indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol 85:1099–1102.
St-Pierre B, Laflamme P, Alarco AM, De Luca V (1998) The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J 14:703–713. doi:10.1046/j.1365-313x.1998.00174.x
Vázquez-Flota F, De Luca V (1998) Developmental and light regulation of desacetoxyvindoline 4-hydroxylase in Catharanthus roseus. Evidence of a multilevel regulatory mechanism. Plant Physiol 117:1351–1361. doi:10.1104/pp. 117.4.1351
St-Pierre B, De Luca V (1995) A cytochrome P-450 monooxygenase catalyzes the first step in the conversion of tabersonine to vindoline in Catharanthus roseus. Plant Physiol 109:131–139. doi:10.1104/pp. 109.1.131
Vázquez-Flota F, De Luca V, Carrillo-Pech M, Canto-Flick A, Miranda-Ham ML (2002) Vindoline biosynthesis is transcriptionally blocked in Catharanthus roseus cell suspension cultures. Mol Biotechnol 22:1–8. doi:10.1385/MB:22:1:001
Aerts RJ, De Luca V (1992) Phytochrome is involved in the light-regulation of vindoline biosynthesis in Catharanthus roseus. Plant Physiol 100:1029–1032. doi:0032-0889/92/100/1029/04/$01.00/0
Aerts R, Gisi D, De Carolis E, De Luca V, Baumann TW (1994) Methyl jasmonate vapor increases the developmentally controlled synthesis of alkaloids in Catharanthus and Cinchona seedlings. Plant J 5:635–643. doi:10.1111/j.1365-313X.1994.00635.x
Sottomayor M, Ros Barceló A (2003) Peroxidase from Catharanthus roseus (L.) G. Don and the biosynthesis of α-3′,4′-anhydrovinblastine: a specific role for a multifunctional enzyme. Protoplasma 222:97–105. doi:10.1007/s00709-003-0003-9
Costa MMR, Hilliou F, Duarte P, Pereira LG, Almeida I, Leech M, Memelink J, Ros Barceló A, Sottomayor M (2008) Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol 146:403–417. doi:10.1104/pp. 107.107060
St-Pierre B, Vazquez-Flota FA, De Luca V (1999) Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11:887–900. doi:10.1105/tpc.11.5.887
Sottomayor M, Lopes Cardoso I, Pereira LG, Ros Barceló A (2004) Peroxidase and the biosynthesis of terpenoid indole alkaloids in the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem Rev 3:159–171. doi:10.1023/B:PHYT.0000047807.66887.09
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for production of plant secondary metabolites. Phytochem Rev 1:13–25. doi:10.1023/A:1015871916833
Zhao J, Verpoorte R (2007) Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: from biochemical processing to metabolic engineering. Phytochem Rev 6:435–457. doi:10.1007/s11101-006-9050-0
Deus B, Zenk MH (1982) Exploitation of plant cells for the production of natural compounds. Biotechnol Bioeng 24:1965–1974. doi:10.1002/bit.260240905
Kurz WGW, Chatson KB, Constabel F (1985) Biosynthesis and accumulation of indole alkaloids in cell suspension cultures of Catharanthus roseus cultivars. In: Neumann KH, Barz W, Reinhard E (eds) Primary and secondary metabolism of plant cell cultures. Springer-Verlag, Berlin, pp 143–153
Naaranlahti T, Ranta VP, Jarho P, Nordstrom M, Lapinjoki SP (1989) Electrochemical detection of indole alkaloids of Catharanthus roseus in high-performance liquid chromatography. Analyst 114:1229–1231. doi:10.1039/AN9891401229
Ganapathi B, Kargi F (1990) Recent advances in indole alkaloid production by Catharanthus roseus (Periwinkle). J Exp Bot 41:259–267. doi:10.1093/jxb/41.3.259
Shukla AK, Shasany AK, Verma RK, Gupta MM, Mathur AK, Khanuja PSP (2010) Influence of cellular differentiation and elicitation on intermediate and late steps of terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Protoplasma 24:35–47. doi:10.1007/s00709-010-0120-1
Endo T, Goodbody AE, Misawa M (1987) Alkaloid production in root and shoot cultures of Catharanthus roseus. Planta Med 53:479–482. doi:10.1055/s-2006-962777
O’Keefe BR, Mahady GB, Gills JJ, Beecher CWW (1997) Stable vindoline production in transformed cell cultures of Catharanthus roseus. J Nat Prod 60:261–264. doi:10.1021/np960703n
Miura Y, Hirata K, Kurano N, Miyamoto K, Uchida K (1988) Formation of vinblastine in multiple shoot culture of Catharanthus roseus. Planta Med 54:18–20. doi:10.1055/s-2006-962321
Bhadra R, Vani S, Shanks JV (1993) Production of indole alkaloids by selected hairy root lines of Catharanthus roseus. Biotechnol Bioeng 41:581–592. doi:10.1002/bit.260410511
Jung KH, Kwak SS, Choi CY, Liu JR (1995) An interchangeable system of hairy root and cell suspension cultures of Catharanthus roseus for indole alkaloid production. Plant Cell Rep 15:51–54. doi:10.1007/BF01690252
Palazón J, Cusidó RM, Gonzalo J, Bonfill M, Morales C, Pinol T (1998) Relation between the amount of rolC gene product and indole alkaloid accumulation in Catharanthus roseus transformed root cultures. J Plant Physiol 153:712–718. doi:35400007196000.0270
Binder BYK, Peebles CAM, Shanks JV, San KY (2009) The effects of UV-B stress on the production of terpenoid indole alkaloids in Catharanthus roseus hairy roots. Biotechnol Prog 25:861–865. doi:10.1002/btpr.97
Pan QF, Chen Y, Wang Q, Yuan F, Xing SH, Tian YS, Zhao JY, Sun XF, Tang KX (2010) Effect of plant growth regulators on the biosynthesis of vinblastine, vindoline and catharanthine in Catharanthus roseus. Plant Growth Reg 60:133–141. doi:10.1007/s10725-009-9429-1
Peebles C, Sander GW, Hughes EH, Peacock R, Shanks JV, San KY (2011) The expression of 1-deoxy-D-xylulose synthase and geraniol-10-hydroxylase or anthranilate synthase increases terpenoid indole alkaloid accumulation in Catharanthus roseus hairy roots. Metab Eng 13:234–240. doi:10.1016/j.ymben.2010.11.005
Tang KX, Liu DH, Wang YL, Cui LJ, Ren WW, Sun XF (2011) Overexpression of transcriptional factor ORCA3 increases the accumulation of catharanthine and vindoline in Catharanthus roseus hairy roots. Rus J Plant Physiol 58:415–422. doi:10.1134/S1021443711030125
van der Heijden R, Verpoorte R, ten Hoopen HJG (1989) Cell and tissue cultures of Catharanthus roseus (L.) G. Don: a literature survey. Plant Cell Tiss Org Cult 18:231–280. doi:10.1007/BF00043397
Zhao J, Hu Q, Guo YQ, Zhu WH (2001) Effects of stress factors, bioregulators, and synthetic precursors on indole alkaloid production in compact callus clusters cultures of Catharanthus roseus. Appl Microbiol Biotechnol 55:693–698. doi:10.1007/s002530000568
Schlatmann JE, Koolhaas CMA, Vinke JL, ten Hoopen HJG, Heijnen JJ (1995) The role of glucose in ajmalicine production by Catharanthus roseus cell cultures. Biotechnol Bioeng 47:525–534. doi:10.1002/bit.260470504
Zhao J, Zhu WH, Hu Q, He XW (2001) Enhanced indole alkaloid production in suspension compact callus clusters of Catharanthus roseus: impacts of plant growth regulators and sucrose. Plant Growth Reg 33:33–41. doi:10.1023/A:1010732308175
Jung KH, Kwak SS, Kim SW, Lee H, Choi CY, Liu JR (1992) Improvement of the catharanthine productivity in hairy root cultures of Catharanthus roseus by using monosaccharides as a carbon source. Biotechnol Lett 14:695–700. doi:10.1007/BF01021645
Almagro L, López-Pérez AJ, Pedreño MA (2010) New method to enhance ajmalicine production in Catharanthus roseus cell cultures based on the use of cyclodextrins. Biotechnol Lett 33:381–385. doi:10.1007/s10529-010-0430-6
Lee CWT, Shuler ML (2000) The effect of inoculum density and conditioned medium on the production of ajmalicine and catharanthine from immobilized Catharanthus roseus cells. Biotechnol Bioeng 67:61–71. doi:10.1002/(SICI)1097-0290(20000105)67:1<61::AID-BIT7>3.0.CO;2-J
Whitmer S, Verpoorte R, Canel C (1998) Influence of auxins on alkaloid accumulation by a transgenic cell line of Catharanthus roseus. Plant Cell Tissue Organ Cult 53:135–141. doi:10.1104/pp. 116.2.853
El-Sayed M, Verpoorte R (2002) Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tiss Organ Cult 68:265–270. doi:10.1023/A:1013968826635
Zhao J, Zhu WH, Hu Q (2001) Effects of light and plant growth regulators on the biosynthesis of vindoline and other indole alkaloids in Catharanthus roseus callus cultures. Plant Growth Reg 33:43–49. doi:10.1023/A:1010722925013
Satdive RK, Fulzele DP, Eapen S (2003) Studies on production of ajmalicine in shake flasks by multiple shoot cultures of Catharanthus roseus. Biotechnol Prog 19:1071–1075. doi:10.1021/bp020138g
Lee CWT, Shuler ML (1991) Different shake flask closures alter gas phase composition and ajmalicine production in Catharanthus roseus cell suspensions. Biotechnol Technol 5:173–178. doi:10.1007/BF00152776
Lee-Parson CWT, Shuler ML (2005) Sparge gas composition affects biomass and ajmalicine production from immobilized cell cultures of Catharanthus roseus. Enzyme MicrobTechnol 37:424–434. doi:10.1016/j.enzmictec.2005.02.016
El-Sayed M, Choi YH, Fréderichrich M, Roytrakul S, Verpoorte R (2004) Alkaloid accumulation in Catharanthus roseus cell suspension cultures fed with stemmadenine. Biotechnol Lett 26:793–798. doi:10.1023/B:BILE.0000025879.53632.f2
Zhao J, Zhu WH, Hu Q (2000) Enhanced ajmalicine production in Catharanthus roseus cell cultures by combined elicitor treatment: from shake-flask to 20-l airlift bioreactor. Biotechnol Lett 22:509–514. doi:10.1023/A:1005616920165. doi:10.1023/A:1005616920165
Morgan JA, Shanks JV (2000) Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol 79:137–145. doi:10.1016/S0168-1656(00)00221-2
Asada M, Shuler ML (1989) Stimulation of ajmalicine production and excretion from Catharanthus roseus: effects of adsorption in situ, elicitors, and alginate immobilization. Appl Microbiol Biotechnol 30:475–481. doi:10.1007/BF00263851
Lee-Parsons CWT, Ertürk S, Tengtrakool J (2004) Enhancement of ajmalicine production in Catharanthus roseus cell cultures with MeJa is dependent on timing and dosage of elicitation. Biotechnol Lett 26:1595–1599. doi:10.1023/B:BILE.0000045825.37395.94
Xu MJ, Dong JF (2005) Effect of nitric oxide on catharanthine production and growth of Catharanthus roseus suspension cells. Biotechnol Bioeng 89:367–371. doi:10.1002/bit.20334
Godoy-Hernández GC, Loyola-Vargas VM (1997) Effect of acetylsalicylic acid on secondary metabolism of Catharanthus roseus tumor suspension cultures. Plant Cell Rep 16:287–290. doi:10.1007/BF01088282
Ramani S, Jayabaskaran C (2008) Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J Mol Signal 3:9–14. doi:10.1186/1750-2187-3-9
Almagro L, Sabater-Jara AB, Belchi-Navarro S, Fernandez-Perez F, Bru R, Pedreño MA (2011) Effect of UV light on secondary metabolite biosynthesis in plant cell cultures elicited with cyclodextrins and methyljasmonate. Plants and environment, 1st edn. Intech, Croatia
Godoy-Hernández GC, Vázquez-Flota FA, Loyola-Vargas VM (2000) The exposure to trans-cinnamic acid of osmotically stressed Catharanthus roseus cells cultured in a 14-L bioreactor increases alkaloid accumulation. Biotechnol Lett 22:921–925. doi:10.1023/A:1005672400219
Smith JI, Smart NJ, Kurz WGW, Misawa M (1987) The use of organic and inorganic compounds to increase the accumulation of indole alkaloids in Catharanthus roseus (L.) G. Don cell suspension cultures. J Exp Bot 38:1501–1506. doi:10.1093/jxb/38.9.1501
Oksman-Caldentey KM, Inzé D (2004) Plant cell factories in the post-genomic era: new ways to produce designer secondary metabolites. Trends Plant Sci 9:1360–1385. doi:10.1016/j.tplants.2004.07.006
Schäfer H, Wink M (2009) Medicinally important secondary metabolites in recombinant microorganisms or plants: progress in alkaloid biosynthesis. Biotechnol J 4:1684–1703. doi:10.1002/biot.200900229
Zárate R, Verpoorte R (2007) Strategies for the genetic modification of the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem Rev 6:475–491. doi:10.1007/s11101-006-9020-6
Lorence A, Verpoorte R (2004) Gene transfer and expression in plants. Methods Mol Biol 267:329–350. doi:10.1385/1-59259-774-2:329
Whitmer S, van der Heijden R, Verpoorte R (2002) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96:193–203. doi:10.1016/S0168-1656(02)00027-5
Wang CT, Liu H, Gao XS, Zhang HX (2010) Overexpression of G10H and ORCA3 in the hairy roots of Catharanthus roseus improves catharanthine production. Plant Cell Rep 29:887–894. doi:10.1007/s00299-010-0874-0
Jaggi M, Kumar S, Sinha AK (2011) Overexpression of an apoplastic peroxidase gene CrPrx in transgenic hairy root lines of Catharanthus roseus. Appl Microbiol Biotechnol 90:1005–1016. doi:10.1007/s00253-011-3131-8
Magnotta M, Murata J, Chen J, De Luca V (2007) Expression of deacetylvindoline-4-O-acetyltransferase in Catharanthus roseus hairy roots. Phytochemistry 68:1922–1931. doi:10.1016/j.phytochem.2007.04.037
van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297. doi:10.1126/science.289.5477.295
Peebles C, Hughes EH, Jacqueline V, Shanks SKY (2009) Transcriptional response of the terpenoid indole alkaloid pathway to the overexpression of ORCA3 along with jasmonic acid elicitation of Catharanthus roseus hairy roots over time. Metab Eng 11:76–86. doi:10.1016/j.ymben.2008.09.002
Hallard D, van der Heijden R, Verpoorte R, Lopez-Cardoso I, Pasquali G, Memelink J, Hoge JHC (1997) Suspension cultured transgenic cells of Nicotiana tabacum expressing tryptophan decarboxylase and strictosidine synthase cDNAs from Catharanthus roseus produce strictosidine upon feeding of secologanin. Plant Cell Rep 17:50–54. doi:10.1007/s002990050350
Hallard D (2000) Transgenic plant cells for the production of indole alkaloids. Ph.D.-thesis, Leiden University, ISBN 90-74538-49-5
Geerlings A, Hallard D, Caballero AM, Cardoso IL (1999) Alkaloid production by a Cinchona officinalis ‘Ledgeriana’ hairy root culture containing constitutive expression constructs of tryptophan decarboxylase and strictosidine synthase cDNAs from Catharanthus roseus. Plant Cell Rep 19:191–196. doi:10.1007/s002990050732
Geerlings A, Redondo FJ, Contin A, Memelink J, van der Heijden R, Verpoorte R (2001) Biotransformation of tryptamine and secologanin into plant terpenoid indole alkaloids by transgenic yeast. Appl Microbiol Biotechnol 56:420–424. doi:10.1007/s002530100663
Shen ZQ, Eisenreich W, Kutchan TM (1998) Bacterial biotransformation of 3 alpha(S)-strictosidine to the monoterpenoid indole alkaloid vallesiachotamine. Phytochemistry 48:293–296. doi:10.1016/S0031-9422(97)01116-3
Acknowledgments
This study was supported by the Fundación Séneca, Agencia de Ciencia y Tecnología de la Región de Murcia en el marco de II PCTRM 2007-10 (08799/PI/08). We are also grateful to the European Cooperation in the field of Scientific and Technical Research (Cost Action FA1006). Almagro L. has a grant from the Ministerio de Ciencia e Innovación.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this entry
Cite this entry
Almagro, L., Sottomayor, M., Angeles Pedreño, M. (2013). Bioproduction of Terpenoid Indole Alkaloids from Catharanthus roseus Cell Cultures. In: Ramawat, K., Mérillon, JM. (eds) Natural Products. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-22144-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-642-22144-6_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-22143-9
Online ISBN: 978-3-642-22144-6
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics