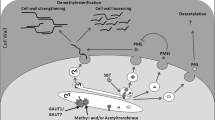
Summary
Leaf architecture is determined by cell shape, size, and density. As plant cells are enclosed by a rigid cell wall, changes to leaf architecture have to occur through downstream genetic systems that induce alterations in (1) cell wall composition, (2) synthesis, assembly, and orientation of cytoskeletal elements and/or (3) the degree of cross-linkage between wall components in response to upstream developmental and environmental cues. This chapter reviews how leaf architecture is influenced by molecular mechanisms that modulate the above wall modification processes. Upstream signaling systems such as salicylic (SA), jasmonic (JA), and gibberellic (GA) acid have significant effects on leaf architecture. GA promotes and JA and SA suppress growth. Leaf architectural changes are brought about by these upstream systems in concert or in an interactive manner, and the associated downstream molecular systems that are involved in executing changes to cell wall properties will be discussed. Evidence will be provided to show that xyloglucan endotransglucosylase/hydrolase and pectin methyltransferase/pectin methylesterase/pectin methylesterase inhibitor systems are key downstream execution points of leaf architectural changes common to different upstream molecular systems. Optimization of leaf architecture maximizes light interception, gas exchange properties, and photosynthesis. In addition, plant growth has been shown to be more sensitive to leaf area than to area-based photosynthesis rate. Therefore, understanding genes and molecular mechanisms that affect cell wall properties and leaf architecture has broader implications in terms of crop improvement, and candidate genes that can be manipulated to optimize leaf architecture in order to maximize net carbon assimilation and plant growth will be proposed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- ABP1 :
-
PUTATIVE AUXIN RECEPTOR AUXIN BINDING PROTEIN
- AIR:
-
alcohol insoluble residue
- AN :
-
ANGUSTIFOLIA
- ARP2/3 :
-
ACTIN RELATED PROTEIN
- BR:
-
brassinosteroids
- BRICK1 :
-
SCAR/WAVE ACTIN-NUCLEATING COMPLEX SUBUNIT
- C:
-
carbon
- CAMTA :
-
CALMODULIN BINDING TRANSCRIPTION ACTIVATOR
- CBF :
-
CRT/DRE BINDING FACTOR
- CBP60G :
-
CALMODULIN BINDING PROTEIN 60G
- CESA :
-
CELLULOSE SYNTHASE
- CGR :
-
COTTON GOLGI-RELATED
- cgr2com:
-
cgr2/3 complemented by CGR2
- CGR2OX:
-
CGR2 overexpression line of Arabidopsis thaliana
- CO2 :
-
carbon dioxide
- CRT:
-
C-repeat
- CSL :
-
CELLULOSE SYNTHASE LIKE
- CYP85A1 :
-
BRASSINOSTEROID-6-OXIDASE
- CYP90C1 :
-
CYTOCHROME P-450 FAMILY STEROID HYDROLASE
- DELLA:
-
PIF transcription factor repressors
- DRE:
-
dehydration responsive element
- ER :
-
ERECTA
- F-actin:
-
actin microfilaments
- FR:
-
far-red
- GA:
-
gibberellic acid
- GT8 :
-
GLYCOSYL TRANSFERASE8
- IAA:
-
auxin
- ICS1 :
-
ISOCHORISMATE SYNTHASE
- IIIe bHLHs:
-
basic helix-loop-helix subgroup IIIe transcription factors
- JA:
-
jasmonic acid
- JAZ:
-
jasmonate ZIM-domain repressors
- jazQ:
-
JAZ quintuple mutation
- LMA:
-
leaf dry mass per unit leaf area
- LMD:
-
leaf mass density
- LNG :
-
LONGIFOLIA
- MAP18 :
-
MICROTUBULE ASSOCIATED PROTEIN18
- MDP40 :
-
MICROTUBULE DESTABILIZING PROTEIN40
- MF:
-
fine actin filament
- MOR1 :
-
MICROTUBULE ORGANIZATION PROTEIN
- MT:
-
microtubule
- MYC2:
-
a basic helix-loop-helix transcription factor
- NAP1 :
-
NCK-ASSOCIATED PROTEIN
- nt:
-
nucleotide
- PCR:
-
polymerase chain reaction
- Pfr:
-
active form of phytochrome that absorbs far-red light
- PHYB :
-
PHYTOCHROME-B
- PIF:
-
phytochrome-interacting factors
- PIN :
-
PIN-FORMED AUXIN EFFLUX CARRIER GENE FAMILY PROTEIN
- PME :
-
PECTIN METHYLESTERASE
- PMEI :
-
PECTIN METHYLESTERASE INHIBITOR
- PMT:
-
pectin methyltransferase
- Pr:
-
inactive form of phytochrome that absorbs red light
- PVX:
-
potato virus X vector
- QUA :
-
QUASIMODO
- R:
-
red
- RhoGAP:
-
negative regulator of ROP
- RhoGEF:
-
Rho-guanine nucleotide exchange factors
- RIC :
-
ROP-INTERACTIVE CRIB MOTIF PROTEIN
- RNA-seq:
-
RNA sequencing
- ROP :
-
RHO-RELATED GTPASE FROM PLANT (RhoGTPase)
- ROT3 :
-
ROTUNDIFOLIA3
- rubisco:
-
ribulose bisphosphate carboxylase oxygenase
- SA:
-
salicylic acid
- SARD1 :
-
SYSTEMIC ACQUIRED RESISTANCE DEFICIENT 1
- SBP-BOX :
-
SQUAMOSA PROMOTER BINDING PROTEIN–LIKE GENE
- S c :
-
chloroplast surface area facing intercellular air spaces per unit leaf area
- SCAR :
-
SUPPRESSOR OF CYCLIC AMP RECEPTOR
- sid2-1 :
-
salicylic acid induction-deficient 2-1
- siRNA:
-
small interfering RNA
- S mes :
-
mesophyll cell surface area facing intercellular air spaces per unit leaf area
- SPIKE :
-
RHOGEF OR DOCK180-TYPE GUANINE NUCLEOTIDE EXCHANGE FACTOR
- SRA1 :
-
RAC1-ASSOCIATED PROTEIN-1
- TMK :
-
TRANSMEMBRANE KINASE SUBFAMILY OF RECEPTOR-LIKE KINASES
- TSD2 :
-
TUMOROUS SHOOT DEVELOPMENT2
- VIGS:
-
virus-induced gene silencing
- VPDB:
-
Vienna-Pee-Dee Belemnite standard
- WAVE :
-
WISKOTT–ALDRICH SYNDROME PROTEIN-FAMILY VERPROLIN HOMOLOGOUS PROTEIN
- XEH :
-
XYLOGLUCAN ENDOHYDROLASE
- XET :
-
XYLOGLUCAN ENDOTRANSGLUCOSYLASE
- XTH :
-
XYLOGLUCAN ENDOTRANSGLUCOSYLASE/HYDROLASE
- XXT :
-
XYLOSYLTRANSFERASE
- δ13CVPDB :
-
ratio of 13C to 12C isotopes in leaf tissue relative to a Vienna-Pee-Dee Belemnite standard
References
Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, Harberd NP (2007) DELLAs contribute to plant photomorphogenesis. Plant Physiol 143:1163–1172
An SH, Sohn KH, Choi HW, Hwang IS, Lee SC, Hwang BK (2008) Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228:61–78
Arioli T, Peng L, Betzner AS, Burn J, Wittke W, Herth W et al (1998) Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279:717–720
Baskin TI (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21:203–222
Basu D, Le J, El-Essal SE, Huang S, Zhang C, Mallery EL et al (2005) DISTORTED3/SCAR2 is a putative Arabidopsis WAVE complex subunit that activates the Arp2/3 complex and is required for epidermal morphogenesis. Plant Cell 17:502–524
Basu D, Le J, Zakharova T, Mallery EL, Szymanski DB (2008) A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc Natl Acad Sci U S A 105:4044–4049
Becnel J, Natarajan M, Kipp A, Braam J (2006) Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and Genevestigator. Plant Mol Biol 61:451–467
Beeckman T, Przemeck GKH, Stamatiou G, Lau R, Terryn N, De Rycke R et al (2002) Genetic complexity of cellulose synthase a gene function in Arabidopsis embryogenesis. Plant Physiol 130:1883–1893
Behringer C, Schwechheimer C (2015) B-GATA transcription factors – insights into their structure, regulation and role in plant development. Front Plant Sci 6:90
Bouton S, Leboeuf E, Mouille G, Leydecker MT, Talbotec J, Granier F et al (2002) QUASIMODO1 encodes a putative membrane-bound glycosyltransferase required for normal pectin synthesis and cell adhesion in Arabidopsis. Plant Cell 14:2577–2590
Bowman JL, Eshed Y, Baum SF (2002) Establishment of polarity in angiosperm lateral organs. Trends Genet 18:134–141
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and Molecular Biology of Plants. Wiley, Somerset
Burton RA, Gibeaut DM, Bacic A, Findlay K, Roberts K, Hamilton A et al (2000) Virus-induced silencing of a plant cellulose synthase gene. Plant Cell 12:691–705
Burton RA, Shirley NJ, King BJ, Harvey AJ, Fincher GB (2004) The CesA gene family of barley. Quantitative analysis of transcripts reveals two groups of co-expressed genes. Plant Physiol 134:224–236
Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A et al (2006) Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-d-glucans. Science 311:1940–1942
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344:1879–1900
Campos ML, Yoshida Y, Major IT, de Oliveira Ferreira D, Weraduwage SM et al (2016) Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat Commun 7:12570
Chaiwanon J, Wang W, Zhu J-Y, Oh E, Wang Z-Y (2016) Information integration and communication in plant growth regulation. Cell 164:1257–1268
Chan J (2012) Microtubule and cellulose microfibril orientation during plant cell and organ growth. J Microsc 247:23–32
Chan Z, Grumet R, Loescher W (2011) Global gene expression analysis of transgenic, mannitol-producing, and salt-tolerant Arabidopsis thaliana indicates widespread changes in abiotic and biotic stress-related genes. J Exp Bot 62:4787–4803
Chapman EJ, Greenham K, Castillejo C, Sartor R, Bialy A, Sun TP, Estelle M (2012) Hypocotyl transcriptome reveals auxin regulation of growth-promoting genes through GA-dependent and -independent pathways. PLoS One 7:9
Chen M-H, Sheng J, Hind G, Handa AK, Citovsky V (2000) Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J 19:913–920
Cho SK, Kim JE, Park JA, Eom TJ, Kim WT (2006) Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett 580:3136–3144
Choe S, Tanaka A, Noguchi T, Fujioka S, Takatsuto S, Ross AS et al (2000) Lesions in the sterol Δ7 reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J 21:431–443
Chou Y-H, Pogorelko G, Zabotina OA (2012) Xyloglucan xylosyltransferases XXT1, XXT2, and XXT5 and the glucan synthase CSLC4 form Golgi-localized multiprotein complexes. Plant Physiol 159:1355–1366
Colebrook EH, Thomas SG, Phillips AL, Hedden P (2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217:67–75
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Craddock C, Lavagi I, Yang Z (2012) New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol 22:492–501
Cunningham SA, Summerhayes B, Westoby M (1999) Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol Monogr 69:569–588
Cutler JM, Rains DW, Loomis RS (1977) The importance of cell size in the water relations of plants. Physiol Plant 40:255–260
de Vries RP, Visser J (2001) Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol Mol Biol Rev 65:497–522
Dijkstra P, Reegen H, Kuiper PJ (1990) Relation between relative growth rate, endogenous gibberellins, and the response to applied gibberellic acid for Plantago major. Physiol Plant 79:629–634
Djakovic S, Dyachok J, Burke M, Frank MJ, Smith LG (2006) BRICK1/HSPC300 functions with SCAR and the ARP2/3 complex to regulate epidermal cell shape in Arabidopsis. Development 133:1091–1100
Doblin MS, Pettolino FA, Wilson SM, Campbell R, Burton RA, Fincher GB et al (2009) A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-β-D-glucan synthesis in transgenic Arabidopsis. Proc Natl Acad Sci U S A 106:5996–6001
Doherty CJ, Van Buskirk HA, Myers SJ, Thomashow MF (2009) Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell 21:972–984
Dwivany FM, Yulia D, Burton RA, Shirley NJ, Wilson SM, Fincher GB et al (2009) The CELLULOSE-SYNTHASE LIKE C (CSLC) family of barley includes members that are integral membrane proteins targeted to the plasma membrane. Mol Plant 2:1025–1039
Ellis B, Daly DC, Hickey LJ, Mitchell JV, Johnson KR, Wilf P, Wing SL (2009) Manual of Leaf Architecture. Cornell University Press, Ithaca
Eriksson EM, Bovy A, Manning K, Harrison L, Andrews J, De Silva J et al (2004) Effect of the colorless non-ripening mutation on cell wall biochemistry and gene expression during tomato fruit development and ripening. Plant Physiol 136:4184–4197
Ferjani A, Horiguchi G, Yano S, Tsukaya H (2007) Analysis of leaf development in fugu mutants of Arabidopsis reveals three compensation modes that modulate cell expansion in determinate organs. Plant Physiol 144:988–999
Filisetti-Cozzi TM, Carpita NC (1991) Measurement of uronic acids without interference from neutral sugars. Anal Biochem 197:157–162
Finlayson SA, Hays DB, Morgan PW (2007) phyB-1 sorghum maintains responsiveness to simulated shade, irradiance and red light : far-red light. Plant Cell Environ 30:952–962
Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621
Folta KM, Pontin MA, Karlin-Neumann G, Bottini R, Spalding EP (2003) Genomic and physiological studies of early cryptochrome 1 action demonstrate roles for auxin and gibberellin in the control of hypocotyl growth by blue light. Plant J 36:203–214
Foo E, Ross JJ, Davies NW, Reid JB, Weller JL (2006) A role for ethylene in the phytochrome-mediated control of vegetative development. Plant J 46:911–921
Frank MJ, Smith LG (2002) A small, novel protein highly conserved in plants and animals promotes the polarized growth and division of maize leaf epidermal cells. Curr Biol 12:849–853
Fredeen AL, Gamon JA, Field CB (1991) Responses of photosynthesis and carbohydrate-partitioning to limitations in nitrogen and water availability in field-grown sunflower. Plant Cell Environ 14:963–970
Fu Y, Li H, Yang Z (2002) The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell 14:777–794
Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z (2005) Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120:687–700
Fu Y, Xu T, Zhu L, Wen M, Yang Z (2009) A ROP GTPase signaling pathway controls cortical microtubule ordering and cell expansion in Arabidopsis. Curr Biol 19:1827–1832
Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T et al (1997) The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell 9:1951–1962
Fujikura U, Horiguchi G, Tsukaya H (2007) Dissection of enhanced cell expansion processes in leaves triggered by a defect in cell proliferation, with reference to roles of endoreduplication. Plant Cell Physiol 48:278–286
Garnier E (1991) Resource capture, biomass allocation and growth in herbaceous plants. Trends Ecol Evol 6:126–131
Garnier E (1992) Growth analysis of congeneric annual and perennial grass species. J Ecol 80:665–675
Giovane A, Balestrieri C, Quagliuolo L, Castaldo D, Servillo L (1995) A glycoprotein inhibitor of pectin methylesterase in kiwi fruit. Purification by affinity chromatography and evidence of a ripening-related precursor. Eur J Biochem 233:926–929
Gommers CMM, Visser EJW, Onge KRS, Voesenek LACJ, Pierik R (2013) Shade tolerance: when growing tall is not an option. Trends Plant Sci 18:65–71
Graham LE, Graham JM, Wilcox LW (2006) Plant biology. Pearson Prentice Hall, New Jersey
Guerriero G, Hausman JF, Cai G (2014) No stress! Relax! Mechanisms governing growth and shape in plant cells. Int J Mol Sci 15:5094–5114
Han Y, Wang W, Sun J, Ding M, Zhao R, Deng S et al (2013) Populus euphratica XTH overexpression enhances salinity tolerance by the development of leaf succulence in transgenic tobacco plants. J Exp Bot 64:4225–4238
Hara Y, Yokoyama R, Osakabe K, Toki S, Nishitani K (2014) Function of xyloglucan endotransglucosylase/hydrolases in rice. Ann Bot 114:1309–1318
Havko N, Major I, Jewell J, Attaran E, Browse J, Howe G (2016) Carbon assimilation and partitioning by jasmonate: an accounting of growth–defense tradeoffs. Plants 5:7
Heldt H-W, Piechulla B (2010) Plant Biochemistry. Academic, London
Held MA, Penning B, Brandt AS, Kessans SA, Yong W, Scofield SR, Carpita NC (2008) Small-interfering RNAs from natural antisense transcripts derived from a cellulose synthase gene modulate cell wall biosynthesis in barley. Proc Natl Acad Sci U S A 105:20534–20539
Held MA, Be E, Zemelis S, Withers S, Wilkerson C, Brandizzi F (2011) CGR3: a Golgi-localized protein influencing homogalacturonan methylesterification. Mol Plant 4:832–844
Hirose F, Inagaki N, Hanada A, Yamaguchi S, Kamiya Y, Miyao A et al (2012) Cryptochrome and phytochrome cooperatively but independently reduce active gibberellin content in rice seedlings under light irradiation. Plant Cell Physiol 53:1570–1582
Hisamatsu T, King RW, Helliwell CA, Koshioka M (2005) The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol 138:1106–1116
Holland N, Holland D, Helentjaris T, Dhugga KS, Xoconostle-Cazares B, Delmer DP (2000) A comparative analysis of the plant cellulose synthase (CesA) gene family. Plant Physiol 123:1313–1324
Honda H, Fisher JB (1978) Tree branch angle: maximizing effective leaf area. Science 199:888–890
Hou X, Lee LY, Xia K, Yan Y, Yu H (2010) DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell 19:884–894
Hu L, Millet DB, Mohr MJ, Wells KC, Griffis TJ, Helmig D (2011) Sources and seasonality of atmospheric methanol based on tall tower measurements in the US Upper Midwest. Atmos Chem Phys 11:11145–11156
Itoh H, Ueguchi-Tanaka M, Sato Y, Ashikari M, Matsuoka M (2002) The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 14:57–70
Jaillais Y, Chory J (2010) Unraveling the paradoxes of plant hormone signaling integration. Nat Struct Mol Biol 17:642–645
Jan A, Yang G, Nakamura H, Ichikawa H, Kitano H, Matsuoka M et al (2004) Characterization of a xyloglucan endotransglucosylase gene that is up-regulated by gibberellin in rice. Plant Physiol 136:3670–3681
Jiang CM, Li CP, Chang JC, Chang HM (2002) Characterization of pectinesterase inhibitor in jelly fig (Ficus awkeotsang Makino) achenes. J Agric Food Chem 50:4890–4894
Kalve S, Fotschki J, Beeckman T, Vissenberg K, Beemster GTS (2014) Three-dimensional patterns of cell division and expansion throughout the development of Arabidopsis thaliana leaves. J Exp Bot 65(22):6385–6397
Karve AA, Jawdy SS, Gunter LE, Allen SM, Yang X, Tuskan GA et al (2012) Initial characterization of shade avoidance response suggests functional diversity between Populus phytochrome B genes. New Phytol 196:726–737
Kawade K, Horiguchi G, Usami T, Hirai Masami Y, Tsukaya H (2013) ANGUSTIFOLIA3 signaling coordinates proliferation between clonally distinct cells in leaves. Curr Biol 23:788–792
Keuskamp DH, Sasidharan R, Vos I, Peeters AJ, Voesenek LA, Pierik R (2011) Blue-light-mediated shade avoidance requires combined auxin and brassinosteroid action in Arabidopsis seedlings. Plant J 67:208–217
Kim G-T, Tsukaya H, Uchimiya H (1998) The ROTUNDIFOLIA3 gene of Arabidopsis thaliana encodes a new member of the cytochrome P-450 family that is required for the regulated polar elongation of leaf cells. Genes Dev 12:2381–2391
Kim GT, Shoda K, Tsuge T, Cho KH, Uchimiya H, Yokoyama R et al (2002) The ANGUSTIFOLIA gene of Arabidopsis, a plant CtBP gene, regulates leaf-cell expansion, the arrangement of cortical microtubules in leaf cells and expression of a gene involved in cell-wall formation. EMBO J 21:1267–1279
Kim G-T, Fujioka S, Kozuka T, Tax FE, Takatsuto S, Yoshida S, Tsukaya H (2005) CYP90C1 and CYP90D1 are involved in different steps in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. Plant J 41:710–721
Kim G-T, Cho K-H (2006) Recent advances in the genetic regulation of the shape of simple leaves. Physiol Plant 126:494–502
Kim Y, Park S, Gilmour SJ, Thomashow MF (2013) Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J 75:364–376
Kim S-J, Held MA, Zemelis S, Wilkerson C, Brandizzi F (2015) CGR2 and CGR3 have critical overlapping roles in pectin methylesterification and plant growth in Arabidopsis thaliana. Plant J 82:208–220
Kost B (2010) Regulatory and cellular functions of plant RhoGAPs and RhoGDIs. In: Yalovsky S, Baluška F, Jones A (eds) Integrated G Proteins Signaling in Plants. Springer, Berlin/Heidelberg, pp 27–48
Kozuka T, Horiguchi G, Kim GT, Ohgishi M, Sakai T, Tsukaya H (2005) The different growth responses of the Arabidopsis thaliana leaf blade and the petiole during shade avoidance are regulated by photoreceptors and sugar. Plant Cell Physiol 46:213–223
Krupkova E, Immerzeel P, Pauly M, Schmulling T (2007) The TUMOROUS SHOOT DEVELOPMENT2 gene of Arabidopsis encoding a putative methyltransferase is required for cell adhesion and co-ordinated plant DEVELOPMENT. Plant J 50:735–750
Lambers H, Chapin F, Pons T (2008) Plant physiological ecology. Springer, NewYork
Leduc N, Roman H, Barbier F, Péron T, Huché-Thélier L, Lothier J et al (2014) Light signaling in bud outgrowth and branching in plants. Plants 3:223
Lee CM, Thomashow MF (2012) Photoperiodic regulation of the C–repeat binding factor (CBF) cold acclimation pathway and freezing tolerance in Arabidopsis thaliana. Proc Natl Acad Sci U S A 109:15054–15059
Lee YK, Kim GT, Kim IJ, Park J, Kwak SS, Choi G, Chung WI (2006) LONGIFOLIA1 and LONGIFOLIA2, two homologous genes, regulate longitudinal cell elongation in Arabidopsis. Development 133:4305–4314
Levesque-Tremblay G, Muller K, Mansfield SD, Haughn GW (2015) HIGHLY METHYL ESTERIFIED SEEDS is a pectin methyl esterase involved in embryo development. Plant Physiol 167:725–737
Li S, Blanchoin L, Yang Z, Lord EM (2003) The putative Arabidopsis arp2/3 complex controls leaf cell morphogenesis. Plant Physiol 132:2034–2044
Li Y, Sorefan K, Hemmann G, Bevan MW (2004) Arabidopsis NAP and PIR regulate actin-based cell morphogenesis and multiple developmental processes. Plant Physiol 136:3616–3627
Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M et al (2007) Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea. Plant Physiol 143:1871–1880
Liu YB, Lu SM, Zhang JF, Liu S, Lu YT (2007) A xyloglucan endotransglucosylase/hydrolase involves in growth of primary root and alters the deposition of cellulose in Arabidopsis. Planta 226:1547–1560
Manning K, Tor M, Poole M, Hong Y, Thompson AJ, King GJ et al (2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38:948–952
Marcotrigiano M (2010) A role for leaf epidermis in the control of leaf size and the rate and extent of mesophyll cell division. Am J Bot 97:224–233
Markovic O, Janecek S (2004) Pectin methylesterases: sequence-structural features and phylogenetic relationships. Carbohydr Res 339:2281–2295
Masle J, Gilmore SR, Farquhar GD (2005) The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 436:866–870
Mathur J (2006) Local interactions shape plant cells. Curr Opin Cell Biol 18:40–46
Mathur J, Hülskamp M (2002) Microtubules and microfilaments in cell morphogenesis in higher plants. Curr Biol 12:R669–R676
Matsui A, Yokoyama R, Seki M, Ito T, Shinozaki K, Takahashi T et al (2005) AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. Plant J 42:525–534
Mazzella MA, Casal JJ, Muschietti JP, Fox AR (2014) Hormonal networks involved in apical hook development in darkness and their response to light. Front Plant Sci 5:52
Miao Y, Li HY, Shen J, Wang J, Jiang L (2011) QUASIMODO 3 (QUA3) is a putative homogalacturonan methyltransferase regulating cell wall biosynthesis in Arabidopsis suspension-cultured cells. J Exp Bot 62:5063–5078
Milla-Moreno EA, McKown AD, Guy RD, Soolanayakanahally RY (2016) Leaf mass per area predicts palisade structural properties linked to mesophyll conductance in balsam poplar (Populus balsamifera L.). Botany 94:225–239
Miura K, Hasegawa PM (2010) Sumoylation and other ubiquitin-like post-translational modifications in plants. Trends Cell Biol 20:223–232
Monsi M (1960) Dry-matter reproduction in plants 1. Schemata of dry-matter reproduction. Bot Mag Tokyo 73:81–90
Moon SY, Zheng Y (2003) Rho GTPase-activating proteins in cell regulation. Trends Cell Biol 13:13–22
Mouille G, Ralet MC, Cavelier C, Eland C, Effroy D, Hematy K et al (2007) Homogalacturonan synthesis in Arabidopsis thaliana requires a Golgi-localized protein with a putative methyltransferase domain. Plant J 50:605–614
Müller K, Levesque-Tremblay G, Bartels S, Weitbrecht K, Wormit A, Usadel B et al (2013a) Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol 161:305–316
Müller K, Levesque-Tremblay G, Fernandes A, Wormit A, Bartels S, Usadel B, Kermode A (2013b) Overexpression of a pectin methylesterase inhibitor in Arabidopsis thaliana leads to altered growth morphology of the stem and defective organ separation. Plant Signal Behav 8:e26464
Nagel OW, Konings H, Lambers H (2001) Growth rate and biomass partitioning of wildtype and low-gibberellin tomato (Solanum lycopersicum) plants growing at a high and low nitrogen supply. Physiol Plant 111:33–39
Nakaya M, Tsukaya H, Murakami N, Kato M (2002) Brassinosteroids control the proliferation of leaf cells of Arabidopsis thaliana. Plant Cell Physiol 43:239–244
Neumetzler L, Humphrey T, Lumba S, Snyder S, Yeats TH, Usadel B et al (2012) The FRIABLE1 gene product affects cell adhesion in Arabidopsis. PLoS One 7:14
Niinemets Ü (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469
Nishitani K, Tominaga R (1992) Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. J Biol Chem 267:21058–21064
Ochoa-Villarreal M, Aispuro-Hernández E, Vargas-Arispuro I, Martínez-Téllez MÁ (2012) Plant cell wall polymers: function, structure and biological activity of their derivatives. In: De Souza Gomes A (ed) Polymerization. InTech, Rijeka. https://doi.org/10.5772/46094 Available from: https://www.intechopen.com/books/polymerization/plant-cell-wall-polymers-function-structure-and-biological-activity-of-their-derivatives
Ogawa S, Toyomasu T, Yamane H, Murofushi N, Ikeda R, Morimoto Y et al (1996) A step in the biosynthesis of gibberellins that is controlled by the mutation in the semi-dwarf rice cultivar tan-ginbozu. Plant Cell Physiol 37:363–368
Ohnishi T, Szatmari AM, Watanabe B, Fujita S, Bancos S, Koncz C et al (2006) C-23 hydroxylation by Arabidopsis CYP90C1 and CYP90D1 reveals a novel shortcut in brassinosteroid biosynthesis. Plant Cell 18:3275–3288
Oikawa PY, Giebel BM, Sternberg Lda S, Li L, Timko MP, Swart PK et al (2011) Leaf and root pectin methylesterase activity and 13C/12C stable isotopic ratio measurements of methanol emissions give insight into methanol production in Lycopersicon esculentum. New Phytol 191:1031–1040
Orfila C, Seymour GB, Willats WG, Huxham IM, Jarvis MC, Dover CJ et al (2001) Altered middle lamella homogalacturonan and disrupted deposition of (1-->5)-α-L-arabinan in the pericarp of Cnr, a ripening mutant of tomato. Plant Physiol 126:210–221
Pear JR, Kawagoe Y, Schreckengost WE, Delmer DP, Stalker DM (1996) Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. Proc Natl Acad Sci U S A 93:12637–12642
Peaucelle A, Louvet R, Johansen JN, Hofte H, Laufs P, Pelloux J, Mouille G (2008) Arabidopsis phyllotaxis is controlled by the methyl-esterification status of cell-wall pectins. Curr Biol 18:1943–1948
Peaucelle A, Braybrook S, Hofte H (2012) Cell wall mechanics and growth control in plants: the role of pectins revisited. Front Plant Sci 3:121
Pierik R, De Wit M, Voesenek LA (2011) Growth-mediated stress escape: convergence of signal transduction pathways activated upon exposure to two different environmental stresses. New Phytol 189:122–134
Pilling J, Willmitzer L, Bucking H, Fisahn J (2004) Inhibition of a ubiquitously expressed pectin methyl esterase in Solanum tuberosum L. affects plant growth, leaf growth polarity, and ion partitioning. Planta 219:32–40
Poorter H, Lambers H (1991) Is interspecific variation in relative growth rate positively correlated with biomass allocation to the leaves? Am Nat 138:1264–1268
Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83:553–559
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588
Qian P, Hou S, Guo G (2009) Molecular mechanisms controlling pavement cell shape in Arabidopsis leaves. Plant Cell Rep 28:1147–1157
Qiu JL, Jilk R, Marks MD, Szymanski DB (2002) The Arabidopsis SPIKE1 gene is required for normal cell shape control and tissue development. Plant Cell 14:101–118
Raiola A, Camardella L, Giovane A, Mattei B, De Lorenzo G, Cervone F, Bellincampi D (2004) Two Arabidopsis thaliana genes encode functional pectin methylesterase inhibitors. FEBS Lett 557:199–203
Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G et al (2011) Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol Plant-Microbe Interact 24:432–440
Reed JW, Nagpal P, Poole DS, Furuya M, Chory J (1993) Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5:147–157
Robert S, Mouille G, Höfte H (2004) The mechanism and regulation of cellulose synthesis in primary walls: lessons from cellulose-deficient Arabidopsis mutants. Cellulose 11:351–364
Rose JK, Braam J, Fry SC, Nishitani K (2002) The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol 43:1421–1435
Sánchez-Rodríguez C, Estévez JM, Llorente F, Hernández-Blanco C, Jordá L, Pagán I et al (2009) The ERECTA receptor-like kinase regulates cell wall-mediated resistance to pathogens in Arabidopsis thaliana. Mol Plant-Microbe Interact 22:953–963
Savaldi-Goldstein S, Chory J (2008) Growth coordination and the shoot epidermis. Curr Opin Plant Biol 11:42–48
Shin YK, Yum H, Kim ES, Cho H, Gothandam KM, Hyun J, Chung YY (2006) BcXTH1, a Brassica campestris homologue of Arabidopsis XTH9, is associated with cell expansion. Planta 224:32–41
Shipley B (2002) Trade-offs between net assimilation rate and specific leaf area in determining relative growth rate: relationship with daily irradiance. Funct Ecol 16:682–689
Shpak ED, Berthiaume CT, Hill EJ, Torii KU (2004) Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131:1491–1501
Sinha N (1999) Leaf development in angiosperms. Annu Rev Plant Physiol Plant Mol Biol 50:419–446
Soolanayakanahally RY, Guy RD, Silim SN, Drewes EC, Schroeder WR (2009) Enhanced assimilation rate and water use efficiency with latitude through increased photosynthetic capacity and internal conductance in balsam poplar (Populus balsamifera L.). Plant Cell Environ 32:1821–1832
Suzuki S, Li L, Sun YH, Chiang VL (2006) The cellulose synthase gene superfamily and biochemical functions of xylem-specific cellulose synthase-like genes in Populus trichocarpa. Plant Physiol 142:1233–1245
Tan H-T, Shirley NJ, Singh RR, Henderson M, Dhugga KS, Mayo GM et al (2015) Powerful regulatory systems and post-transcriptional gene silencing resist increases in cellulose content in cell walls of barley. BMC Plant Biol 15:62
Tenhaken R (2015) Cell wall remodeling under abiotic stress. Front Plant Sci 5:771
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57:343–354
Tillman D (1991) Relative growth rates and plant allocation patterns. Am Nat 138:1269–1275
Tosens T, Niinemets Ü, Vislap V, Eichelmann H, Castro Díez P (2012) Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ 35:839–856
Tsuge T, Tsukaya H, Uchimiya H (1996) Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L). Heynh Development 122:1589–1600
Tsukaya H (1998) Genetic evidence for polarities that regulate leaf morphogenesis. J Plant Res 111:113–119
Tsukaya H (2002) The leaf index: heteroblasty, natural variation, and the genetic control of polar processes of leaf expansion. Plant Cell Physiol 43:372–378
Tsukaya H, Kozuka T, Kim G-T (2002) Genetic control of petiole length in Arabidopsis thaliana. Plant Cell Physiol 43:1221–1228
Utrillas MJ, Alegre L (1997) Impact of water stress on leaf anatomy and ultrastructure in Cynodon dactylon (L.) Pers. under natural conditions. Int J Plant Sci 158:313–324
Van Volkenburgh E, Boyer JS (1985) Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol 77:190–194
Verica JA, Medford JI (1997) Modified MER15 expression alters cell expansion in transgenic Arabidopsis plants. Plant Sci 125:201–210
Villagarcia H, Morin A-C, Shpak ED, Khodakovskaya MV (2012) Modification of tomato growth by expression of truncated ERECTA protein from Arabidopsis thaliana. J Exp Bot 63:6493–6504
Villar R, Ruiz-Robleto J, Ubera JL, Poorter H (2013) Exploring variation in leaf mass per area (LMA) from leaf to cell: an anatomical analysis of 26 woody species. Am J Bot 100:1969–1980
Volpi C, Janni M, Lionetti V, Bellincampi D, Favaron F, D’Ovidio R (2011) The ectopic expression of a pectin methyl esterase inhibitor increases pectin methyl esterification and limits fungal diseases in wheat. Mol Plant-Microbe Interact 24:1012–1019
Wang X, Zhang J, Yuan M, Ehrhardt DW, Wang Z, Mao T (2012) Arabidopsis microtubule destabilizing protein40 is involved in brassinosteroid regulation of hypocotyl elongation. Plant Cell 24:4012–4025
Weller JL, Hecht V, Vander Schoor JK, Davidson SE, Ross JJ (2009) Light regulation of gibberellin biosynthesis in pea is mediated through the COP1/HY5 pathway. Plant Cell 21:800–813
Weraduwage S, Chen J, Anozie FC, Morales A, Weise SE, Sharkey TD (2015) The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front Plant Sci 6:167
Weraduwage SM, Kim S-J, Renna L, Anozie FC, Sharkey TD, Brandizzi F (2016) Pectin methylesterification impacts the relationship between photosynthesis and plant growth in Arabidopsis thaliana. Plant Physiol 171:833–848
Whittington AT, Vugrek O, Wei KJ, Hasenbein NG, Sugimoto K, Rashbrooke MC, Wasteneys GO (2001) MOR1 is essential for organizing cortical microtubules in plants. Nature 411:610–613
Wietholter N, Graessner B, Mierau M, Mort AJ, Moerschbacher BM (2003) Differences in the methyl ester distribution of homogalacturonans from near-isogenic wheat lines resistant and susceptible to the wheat stem rust fungus. Mol Plant-Microbe Interact 16:945–952
Williamson RE, Burn JE, Birch R, Baskin TI, Arioli T, Betzner AS, Cork A (2001) Morphology of rsw1, a cellulose-deficient mutant of Arabidopsis thaliana. Protoplasma 215:116–127
Wood PJ, Siddiqui IR (1971) Determination of methanol and its application to measurement of pectin ester content and pectin methylesterase activity. Anal Biochem 39:418–428
Wolf S, Mouille G, Pelloux J (2009) Homogalacturonan methyl-esterification and plant development. Mol Plant 2:851–860
Xiao C, Anderson CT (2013) Roles of pectin in biomass yield and processing for biofuels. Front Plant Sci 4:67
Xu T, Wen M, Nagawa S, Fu Y, Chen JG, Wu MJ et al (2010) Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143:99–110
Yang Y, Karlson D (2012) Effects of mutations in the Arabidopsis cold shock domain protein 3 (AtCSP3) gene on leaf cell expansion. J Exp Bot 63:4861–4873
Yang DL, Yao J, Mei CS, Tong XH, Zeng LJ, Li Q et al (2012) Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc Natl Acad Sci U S A 109:E1192–E1200
Yokoyama R, Nishitani K (2001) A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of Arabidopsis. Plant Cell Physiol 42:1025–1033
Yokoyama R, Rose JK, Nishitani K (2004) A surprising diversity and abundance of xyloglucan endotransglucosylase/hydrolases in rice. Classification and expression analysis. Plant Physiol 134:1088–1099
Yoshikawa T, Eiguchi M, Hibara K-I, Ito J-I, Nagato Y (2013) Rice SLENDER LEAF 1 gene encodes cellulose synthase-like D4 and is specifically expressed in M-phase cells to regulate cell proliferation. J Exp Bot 64:2049–2061
Acknowledgments
We are grateful to Drs. Sean E. Weise, (Department of Biochemistry and Molecular Biology), Cliff Foster (the Cell Wall Facility, Great Lakes Bioenergy Research Center), Alicia Withrow and Melinda Frame (Center for Advanced Microscopy) of Michigan State University (East Lansing, MI), and to Dr. Suvankar Chakraborty (Stable Isotope Ratio Facility for Environmental Research) of the University of Utah (Salt Lake City, UT) for their support. We also wish to thank Jim Klug and Cody Keilen (Growth Chamber Facility) of Michigan State University for their assistance and all members of the Brandizzi, Thomashow, Howe, and Sharkey labs for their support. Funding for this research was provided by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U. S. Department of Energy (award number DE-FG02-91ER20021) and in part by the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494). Partial salary support for MT, GH, TDS, and FB came from Michigan AgBioResearch.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Weraduwage, S.M. et al. (2018). Molecular Mechanisms Affecting Cell Wall Properties and Leaf Architecture. In: Adams III, W., Terashima, I. (eds) The Leaf: A Platform for Performing Photosynthesis. Advances in Photosynthesis and Respiration, vol 44. Springer, Cham. https://doi.org/10.1007/978-3-319-93594-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-93594-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93592-8
Online ISBN: 978-3-319-93594-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)