Abstract
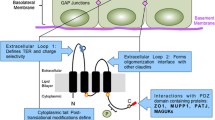
Dysadherin, a small regulator of Na+/K+-ATPase and a cell membrane glycoprotein, is associated with cancer metastasis. However, its role in metastasis is largely unknown. In this review we highlight the role of this recently identified protein in cancer progression. Dysadherin has been suggested to affect cancer progression by downregulating E-cadherin or by upregulating the chemokine production. Overexpression of dysadherin alters trans epithelial resistance (TER) indicating it’s effect on paracellular permeability. Additional findings suggest that dysadherin also affects extracellular matrix. The expression of dysadherin can influence both the tumor cell as well as the cell matrix. Recent findings strongly suggest that dysadherin expression as an independent prognostic indicator of metastasis. Thus, dysadherin can be used as a molecular target for identification as well as prevention of cancer.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ino Y, Gotoh M, Sakamoto M et al (2002) Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci 99:365–370
Lubarski I, Pihakaski-Maunsbach K, Karlish SJ et al (2005) Interaction with the Na+/K + -ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem 280:37717–37724
Geering K (2006) FXYD proteins: new regulators of Na+/K+-ATPase. Am J Physiol 290:F241–F250
Tsuiji H, Takasaki S, Sakamoto M et al (2003) Aberrant O-glycosylation inhibits stable expression of dysadherin, a carcinoma-associated antigen, and facilitates cell-cell adhesion. Glycobiology 13:521–527
Fu X, Kamps MP (1997) E2a-Pbx1 induces aberrant expression of tissue-specific and developmentally regulated genes when expressed in NIH 3T3 fibroblasts. Mol Cell Biol 17:1503–1512
Bild AH, Yao G, Chang JT et al (2006) Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439:353–357
Robinson M, Jiang P, Cui J et al (2003) Global genechip profiling to identify genes responsive to p53-induced growth arrest and apoptosis in human lung carcinoma cells. Cancer Biol Ther 2:406–415
Gabrielli NM, Veiga MF, Matos ML et al (2011) Expression of dysadherin in the human male reproductive tract and in spermatozoa. Fertil Steril 96:554–561
Shimamura T, Yasuda J, Ino Y et al (2004) Dysadherin expression facilitates cell motility and metastatic potential of human pancreatic cancer cells. Cancer Res 64:6989–6995
Nam JS, Kang MJ, Suchar AM et al (2006) Chemokine (C-C motif) ligand 2 mediates the prometastatic effect of dysadherin in human breast cancer cells. Cancer Res 66:7176–7184
Yanagihara K, Tanaka H, Takigahira M et al (2004) Establishment of two cell lines from human gastric scirrhous carcinoma that possess the potential to metastasize spontaneously in nude mice. Cancer Sci 95:575–582
Maehata Y, Hirahashi M, Aishima S et al (2011) Significance of dysadherin and E-cadherin expression in differentiated-type gastric carcinoma with submucosal invasion. Hum Pathol 42:558–567
Sato H, Ino Y, Miura A et al (2003) Dysadherin: expression and clinical significance in thyroid carcinoma. J Clin Endocrinol Metab 88:4407–4412
Colamaio M, Calì G, Sarnataro D et al (2012) Let-7a down-regulation plays a role in thyroid neoplasias of follicular histotype affecting cell adhesion and migration through its ability to target the FXYD5 (Dysadherin) gene. J Clin Endocrinol Metab 97:E2168–F2178
Kang MJ, Suchar AM, Shimamura T et al (2006) Chemokine (C-C motif) ligand 2 mediates the prometastatic effect of dysadherin in human breast cancer cells. Cancer Res 66:7176–7184
Saji H, Koike M, Yamori T et al (2001) Significant correlation of monocyte chemoattractant protein-1 expression with neovascularization and progression of breast carcinoma. Cancer 92:1085–1091
Ohta M, Kitadai Y, Tanaka S et al (2003) Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human gastric carcinomas. Int J Oncol 22:773–778
Youngs SJ, Ali SA, Taub DD et al (1997) Chemokines induce migrational responses in human breast carcinoma cell lines. Int J Cancer 71:257–266
Shimamura T, Sakamoto M, Ino Y et al (2003) Dysadherin overexpression in pancreatic ductal adenocarcinoma reflects tumor aggressiveness: relationship to e-cadherin expression. J Clin Oncol 21:659–667
Lubarski I, Asher C, Garty H (2011) FXYD5 (dysadherin) regulates the paracellular permeability in cultured kidney collecting duct cells. Am J Physiol 301:F1270–F1280
Lubarski I, Asher C, Garty H (2014) Modulation of cell polarization by the Na+/K+-ATPase-associated protein FXYD5 (dysadherin). Am J Physiol 306:C1080–C1088
Vagin O, Tokhtaeva E, Sachs G (2006) The role of the beta1 subunit of the Na, K-ATPase and its glycosylation in cell-cell adhesion. J Biol Chem 281:39573–39587
Park JR, Kim RJ, Lee YK et al (2011) Dysadherin can enhance tumorigenesis by conferring properties of stem-like cells to hepatocellular carcinoma cells. J Hepatol 54:122–131
Tamura M, Ohta Y, Tsunezuka Y et al (2005) Prognostic significance of dysadherin expression in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 130:740–745
Shimada Y, Yamasaki S, Hashimoto Y et al (2004) Clinical significance of dysadherin expression in gastric cancer patients. Clin Cancer Res 10:2818–2823
Nakanishi Y, Akimoto S, Sato Y et al (2004) Prognostic significance of dysadherin expression in tongue cancer: immune histochemical analysis of 91 cases. Appl Immunohistochem Mol Morphol 12:323–328
Batistatou A, Charalabopoulos AK, Scopa CD et al (2006) Expression patterns of dysadherin and E-cadherin in lymph node metastases of colorectal carcinoma. Virchows Arch 448:763–767
Kyzas PA, Stefanou D, Batistatou A et al (2006) Dysadherin expression in head and neck squamous cell carcinoma: association with lymphangiogenesis and prognostic significance. Am J Surg Pathol 30:185–193
Crambert G, Geering K (2003) FXYD proteins: new tissue-specific regulators of the ubiquitous Na+/K+-ATPase. Sci STKE 2003:RE1
Kometiani P, Li J, Gnudi L et al (1998) Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem 273:15249–1556
Mohammadi K, Kometiani P, Xie Z et al (2001) Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem 276:42050–42056
Barwe SP, Anilkumar G, Moon SY et al (2005) Novel role for Na+/K+-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol Biol Cell 16:1082–1094
Lee YK, Lee SY, Park JR et al (2012) Dysadherin expression promotes the motility and survival of human breast cancer cells by AKT activation. Cancer Sci 103:1280–1289
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Dey, K., Garty, H., Chakraborti, S. (2016). Emerging Role of Dysadherin in Metastasis. In: Chakraborti, S., Dhalla, N. (eds) Regulation of Membrane Na+-K+ ATPase. Advances in Biochemistry in Health and Disease, vol 15. Springer, Cham. https://doi.org/10.1007/978-3-319-24750-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-24750-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24748-9
Online ISBN: 978-3-319-24750-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)