Abstract
Cross-sectional imaging techniques including magnetic resonance tomography (MRI) and computed tomography (CT) have experienced rapid development in the last decades and play a key role today in imaging benign and malignant colorectal disease, facilitating risk stratification, and procedural planning. In particular, visualization of the intestinal wall and adjacent structures enables detection of extraluminal and extraintestinal pathologies and complications.
This chapter systematically summarizes the most common benign and malignant conditions of the colon and rectum that can be diagnosed by CT and MRI.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Benign and malignant diseases of the colon and rectum
- Rectal cancer
- Computed tomography
- Magnetic resonance imaging
-
To provide an overview of the most common benign and malignant conditions of the colon and rectum on cross-sectional imaging (CT and MRI).
-
To understand the current role of cross-sectional imaging in the detection, characterization, and differentiation of colorectal diseases.
4.1 Benign Diseases of the Colon and Rectum
4.1.1 Inflammatory Diseases of the Colon and Rectum
CT is a valuable diagnostic tool for the detection and characterization of different inflammatory conditions of the colon, including appendicitis, diverticulitis, epiploic appendagitis, chronic inflammatory bowel diseases (IBDs), as well as infectious and non-infectious colitis. CT plays an important role in detection of acute conditions including extraluminal complications and extraintestinal manifestations of inflammatory bowel disease. Despite the significant overlap in imaging findings of inflammatory bowel diseases, their findings may differ in their primary localization within the gastrointestinal tract, length of segmental involvement, degree of wall thickening, mural enhancement pattern, and extraintestinal involvement. Therefore, understanding of leading disease patterns and specific imaging features can allow accurate diagnosis.
4.1.1.1 Chronic Inflammatory Bowel Diseases
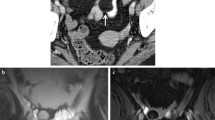
Inflammatory bowel diseases (IBD) are a group of chronic disorders that cause relapsing inflammation in the gastrointestinal tract and comprise three major subgroups of Crohn’s disease, ulcerative colitis, and unclassified. Environmental changes, genetic factors, intestinal microbiota alterations, and immune system deregulation contribute to the initiation and progression of inflammation and subsequent fibrosis [1]. Despite of the considerable overlap between the imaging findings in Crohn’s disease and ulcerative colitis, there are often certain features that can help differentiate them (Table 4.1, see also Fig. 4.1) [2].
A 24-year-old female patient with known history of Crohn’s disease. Axial contrast-enhanced CT with positive oral contrast (a), axial (b), and coronal (d) contrast-enhanced MR-enterography images with neutral oral contrast show stratified mural thickening with hyperenhancement of the terminal ileum (arrows) and presacral abscess formation (c, e) (arrows). Findings are consistent with active inflammatory Crohn’s disease
4.1.1.2 Infectious Colitis
Infectious colitis, as its name suggests, is caused by an infection due to bacterial, viral, fungal, or parasitic agents, leading to inflammation of the colon. Although cross-sectional imaging is not the primary diagnostic tool, and imaging findings are often non-specific, standard abdominal CT may be required to assess disease extent and severity, extraluminal complications, and especially to rule out other causes of acute abdomen [3]. Typical imaging findings regardless of the infective cause are: diffuse wall thickening with homogeneous enhancement, pericolonic fat stranding, gas-fluid levels, and ascites [3].
4.1.1.2.1 Pseudomembranous Colitis
Pseudomembranous colitis is an acute, potentially life-threatening nosocomial infectious colitis caused by toxins produced by an unopposed proliferation of Clostridium difficile bacteria. In recent years, it has become a significant clinical problem, mostly due to the increased use of prophylactic and broad-spectrum antibiotics. Imaging features include marked wall thickening (which is usually more extensive compared to other infectious and non-infectious colitis), low-attenuation mural thickening corresponding to mucosal and submucosal edema, the “accordion sign” (oral contrast media trapped between the thickened colon wall folds), and the “target sign” (or “double halo sign”) (Fig. 4.2). Extracolonic features include ascites and pericolonic stranding, which may be relatively mild compared to the degree of colon wall thickening. Most commonly, the entire colon is affected. In severe cases, complications like intramural gas formation (pneumatosis coli), toxic megacolon, and perforation (pneumoperitoneum) may occur [4].
Composed figure shows different types of inflammatory colitis (a, c, e, g: axial images, b, d, f, h: coronal images). (a, b) A 77-years-old female patient with abdominal pain. Images show distension, wall thickening, and submucosal edema with surrounding stranding involving the colon ascendens (arrows) without occlusion of the mesenterial arteries (not shown). Colonoscopy confirmed ischemic colitis. (c, d) A 59-year-old male patient with history of allogeneic stem cell transplantation presents with sepsis and intestinal bleeding. Images show diffuse bowl wall thickening with submucosal edema affecting the entire non-dilated small and large bowl (arrows). Given the medical history and other clinical findings including biopsy, bowel graft versus host disease was the final diagnosis. (e, f) A 22-year-old female patient with sepsis and long-term treatment at the intensive care unit presents with abdominal distension and diarrhea. Images show thick-walled, mildly dilated, fluid-filled colon with mucosal enhancement and minor pericolic stranding. The entire colon is involved (pancolitis, arrows). As the stool test for Clostridium difficile toxin was positive, pseudomembranous colitis was the final diagnosis. (g, H) A 71-year-old male with metastatic melanoma and known drug-induced pneumonitis under immune-checkpoint-inhibitor therapy (ICI; Nivolumab) presents with new-onset of gastrointestinal bleeding. Images show extensive wall thickening and enhancement of the sigmoid colon (arrows) with surrounding fat stranding and diffuse inflammatory pericolic formation including pneumoperitoneum due to perforation. Perforated ICI-induced colitis was the final clinical diagnosis
4.1.1.3 Non-infectious Colitis
Non-infectious colitis refers to the heterogeneous group of colonic inflammation caused by various causes other than infections (pathogenic organisms), for example, ischemic, drug-induced or immune-mediated.
4.1.1.3.1 Ischemic Colitis
Ischemic colitis is a condition in which inflammatory injury of the colon results from interruption and/or insufficient blood supply. It is more likely to occur in the elderly with atherosclerotic disease and/or low-flow state (e.g., due to heart disease). Low-flow state and non-occlusive vessel disease may lead to ischemic colitis in watershed areas while complete vessel occlusion produces an involvement of the dependent vascular territory (e.g., in the territory of the superior mesenteric artery). Imaging findings are mostly non-specific: uniform bowel wall thickening, “target sign” (low-density ring of submucosal edema between enhancing mucosa and serosa), bowel dilatation, pneumatosis coli (in severe cases), pericolic fluid or fat stranding, mesenteric edema, and/or asities (Fig. 4.2). Multiphase CT angiography has to be performed to identify the level of vessel occlusion and procedural planning.
4.1.1.3.2 Drug-Induced Colitis
The dramatic increase in pharmaceutical medical therapies (e.g., immune-modulating therapies with biologics, chemotherapeutics, nonsteroidal anti-inflammatory drugs) has led to an increased frequency of gastrointestinal adverse effects. Medical history and clinical presentation supported by imaging findings are the key to the diagnosis. Cross-sectional imaging may be required for the assessment of (peri-)colonic involvement, associated complications and to exclude other causes of acute abdomen (e.g., ischemic causes) [5]. Imaging findings are generally based on those seen in other infectious and non-infectious colitis (Fig. 4.2).
4.1.1.3.3 Neutropenic Colitis
Neutropenic colitis (also known as typhlitis) is a severe necrotizing inflammation occurring primarily in neutropenic patients. It mostly originates in the cecum and extends to the ascending colon, appendix, or terminal ileum [6]. As morphologic imaging findings are similar to that of other infectious and non-infectious colitis, medical history (e.g., immunodeficiency) is necessary to establish the diagnosis.
4.1.1.3.4 Radiation Colitis and Proctitis
Radiation colitis is the inflammatory injury of the colon and rectum caused by radiation therapy, which may occur between 6 months to 5 years after treatment. Depending on the onset, radiation colitis may be classified as acute or chronic. Cross-sectional imaging may be indicated for the assessment of extracolonic involvement and other complications. Imaging findings in the acute phase include non-specific wall thickening and pericolonic stranding; in the chronic phase, short or long strictures, colonic lumen narrowing, ulcerations, and/or fistulas may be present [7].
4.1.1.3.5 Graft-Versus-Host Disease
Intestinal graft-versus-host disease (GvHD) is a common, potentially life-threatening complication after hematopoietic stem cell transplantation, which may affect the entire gastrointestinal tract (large bowel involvement is present in ~25% of cases). Imaging findings are non-specific and include: moderate bowel wall thickening with mucosal enhancement, mesenteric edema, vascular engorgement, and/or pneumatosis intestinalis in severe cases (Fig. 4.2) [8].
Key Point
CT plays an essential role in the detection and characterization of inflammatory conditions, including extraluminal complications and extraintestinal manifestations.
4.1.2 Diverticular Disease and Diverticulitis
Diverticular disease is one of the most common gastroenterological disorders in the Western world. In case of acute abdomen and suspicious diverticular disease, ultrasound is routinely followed by CT, further clinical decision-making, and risk stratification. Based on the classification of diverticular disease (CDD), a differentiation can be made between uncomplicated (type 1), complicated (type 2), and chronic (type 3) diverticular disease (Fig. 4.3) [9]. In this context, CT allows the detection of associated microabscesses, macroabscesses, and free perforation as they determine the further therapeutic approach.
(a) A 56-year-old asymptomatic patient with uncomplicated diverticular disease. The axial contrast-enhanced CT image shows multiple diverticula (arrow) of the sigmoid without associated inflammation. (b) A 63-year-old with intermittent pain localized in the left lower abdomen and elevated blood inflammatory markers. The contrast-enhanced axial CT image after the administration of positive rectal contrast shows bowel wall thickening and fat stranding (arrow). Findings are consistent with acute complicated diverticulitis with phlegmonous peridiverticulitis Type 1b. (c) A 60-year-old patient with severe abdominal pain, located in the left lower abdomen, fever, nausea, and elevated blood inflammatory markers. The contrast-enhanced axial CT image after the administration of positive rectal contrast material shows diverticulitis with bowel wall thickening, fat stranding, and covered perforation with small abscess (≤1 cm) and minimal paracolic air (arrow). Findings are consistent with acute complicated diverticulitis Type 2a. (d) A 57-year-old patient with severe abdominal pain, fever, nausea, and elevated blood inflammatory markers. Axial CT image shows acute complicated diverticulitis with phlegmonous peridiverticulitis and paracolic abscess (>1 cm, arrow). Findings are consistent with acute complicated diverticulitis Type 2b
Key Point
CT is the method of choice to evaluate diverticulitis and allows accurate classification and guide treatment.
4.1.3 Benign Mucosal Colonic Polyp
Since the majority of colorectal cancer are believed to arise within benign adenomatous polyps that develop slowly over many years following the “adenoma to carcinoma” sequence, they are the primary target lesions for colorectal screening. Cross-sectional imaging with introduction of virtual colonoscopy (CT and MRI colonography) are promising techniques and play an increasingly important role in both symptomatic and screening patients for the selection of the appropriate therapeutic procedure (see the Abstract Book IDKD 2018).
Key Point
Cross-sectional imaging techniques with the introduction of virtual colonoscopy are promising techniques and are playing an increasing role.
4.2 Malignant Diseases of the Colon and Rectum
4.2.1 Rectal Cancer
Colorectal cancer is the third most common cancer in men and the second most common in women [10]. Nowadays, rectal MRI plays a leading role in the evaluation of rectal cancer, especially in primary local staging and assessment of response to chemotherapeutic treatment.
4.2.1.1 Elective Rectal Cancer Staging
In primary staging (pre-operative setting), MRI is important for the evaluation of tumor location and morphology, T and N category, involvement of the mesorectal fascia (MRF), extramural vascular invasion (EMVI), mucin content, and involvement of the pelvic sidewall and anal sphincter complex (Fig. 4.4). Therefore, rectal MRI is particularly performed for (1) selecting patients with locally advanced rectal cancer who are suitable for treatment with neoadjuvant chemotherapy; (2) guiding surgical planning; and (3) identifying poor prognostic factors, including EMVI, mucin content, and CRM status [10]. The prognosis of rectal cancer is directly related to mesorectal tumor infiltration and circumferential resection margins (CRMs).
Rectal MR images (axial T2-weighted images) show different tumor stages from four different patients. (a) The axial T2-weighed image shows a tumor within the middle rectum infiltrating the muscularis propria (T2). (b) The axial T2-weighted image shows a tumor within the middle rectum with infiltration beyond the muscularis propria (T3c), with negative MRF infiltration and positive EMVI. (c, d) The axial and coronal T2-weighted images show a tumor within the low rectum infiltrating beyond the muscularis propria and invading the external sphincter, internal sphincter complex, and the levator ani muscle (T4b)
Key Point
Rectal MRI plays a key role in local staging of rectal cancer and allows selection of an appropriate treatment strategy. Moreover, it allows to identify poor prognostic factors including MRF and EMVI.
4.2.2 Colon Cancer
Colon cancer is the fourth most commonly diagnosed cancer worldwide and the fifth deadliest, representing 5.8% of all cancer deaths [11]. Its incidence is 3 to 4 times higher in developed countries, making it a marker of socioeconomic development [11].
The diagnosis of colon cancer is either driven by symptoms or screening. Optical colonoscopy is the diagnostic gold standard, with detection rates of (pre)cancerous lesions >95% [12]. CT colonography may be a good alternative, particularly in patients with structural problems or comorbidities, and a good adjunct to incomplete examinations, with comparable sensitivity for lesions >10 mm [13].
Clinical staging of colorectal cancer is the most important predictor of survival and relies on the TNM system proposed by the AJCC/UICC, which is based on the pathologic analysis of the resected specimen [14]. Imaging plays an essential role, not only for surgery planning in eligible patients, but also for distant staging, detection of pre- and postoperative complications, and oncologic follow-up.
4.2.2.1 Elective Colon Cancer Staging
CT is the mainstay for colon cancer staging, but accurate T and N staging has always been a challenge and MRI has not demonstrated better results [15, 16]. Given surgery remains primary curative treatment for all TN stages, more than getting the T and N stages right, the radiologist should provide the multidisciplinary team with all the relevant information for a successful curative surgery or, alternatively, with the detailed baseline information to monitor systemic treatment. In the absence of IV contrast contraindications, a weight and concentration-adjusted acquisition in the portal venous phase of enhancement should be sufficient, oral contrast being considered unnecessary by the great majority of experts [17].
Most colon cancers present as a polyp (Fig. 4.5) or as an asymmetrical or concentrical wall thickening, the latter with lumen caliber reduction and loss of the normal layered appearance of the bowel wall (Fig. 4.6). Relative enhancement varies, most non-mucinous tumors being hyper to isoenhancing (78%) (Figs. 4.5 and 4.6) and most mucinous tumors being iso to hypoenhancing (84%) compared to adjacent bowel wall (Fig. 4.7) [18]. Enhancement pattern is usually heterogeneous, particularly in mucinous tumors (Fig. 4.7). Intratumoral calcification is unusual but relatively more frequent in mucinous tumors [18].
A 66-year-old female patient referred for colon cancer staging after screening colonoscopy detected a small, biopsy conformed, adenocarcinoma. (a) Axial and (b) sagittal cropped CT images acquired on the portal venous phase of enhancement depict a 10 mm polypoid, homogeneously hyperenhancing, lesion of the transverse colon (red arrow). Patient underwent laparoscopic right hemicolectomy and pathology revealed a G1 pT2N0 tumor
An 81-year-old male patient referred for colon cancer staging after screening colonoscopy revealed an ulcerated tumor of the transverse colon. (a) Axial, (b) oblique sagittal, and (c) oblique coronal cropped CT images acquired on the portal venous phase of enhancement. An asymmetrical hyperenhancing, mildly heterogeneous, wall thickening at the hepatic flexure of the colon is observed, associated with focal lumen caliber reduction. Although clinically staged as T2, right hemicolectomy revealed a pT3 N0 specimen
Male patient, 50 years of age, presenting with vomiting and epigastric pain with 2-month duration. Endoscopy found no abnormalities. Colonoscopy revealed a bulky, ulcerated, and stenosing lesion of the ascending colon, which could not be passed with the colonoscope. (a) Coronal maximum intensity projection, (b) axial, and (c) axial maximum intensity projection portal venous phase CT images depicting an 8 cm bulky, irregular, heterogeneous lesion (red arrows) involving the cecum and ascending colon, with areas of hyper-, iso-, and hypoenhancement. In (c), the colic arteries cross the superior mesenteric vein posteriorly (blue arrow), an important information for laparoscopic surgery planning. Laparoscopic right hemicolectomy revealed a mucinous pT3N0 specimen
No reliable radiologic lymph node involvement criteria have been established so far although several have been investigated [16, 19].
The success of curative R0 resection relies on detailed imaging delineation of tumor boundaries, including any involved surrounding organs or structures (Fig. 4.8). Attention should be paid to other colon segments, particularly proximal to tumor in incomplete colonoscopies, not to miss additional lesions. Other details that matter for the selection of the best surgical approach include: specific tumor location, lesion size, and the particularities of mesenteric vascular anatomy, for which multiplanar reformations and maximum intensity projections, especially in coronal plane, may be quite illustrative [20].
A 84-year-old patient presenting with nausea and vomiting. (a) Coronal and (b) axial CT images acquired on the portal venous phase of enhancement. Bulky, circumferential, heterogeneously iso- to hyperenhancing lesion of the ascending colon (red arrows) invading the root of the mesentery, the head of the pancreas, and the 1st, 2nd, and 3rd portions of the duodenum (red arrows), causing severe stenosis with pronounced upstream dilatation—notice the distended stomach (*). The lesion was stenotic itself and could not be passed with the colonoscope, but no upstream small bowel distention was observed because of the stenosing effect on the duodenum. Peritoneal carcinomatosis is visible in the pelvis (blue arrows in a), as are bulky, retroperitoneal lymphadenopathies (green arrows in b)
4.2.2.2 Colon Cancer Presenting as Acute Abdomen
Colon cancer may present as a surgical emergency in up to 40% of cases, which occurs more frequently in the elderly population [21]. Obstruction and perforation, the most common presentations, are considered high-risk features and are linked to poorer recurrence-free survival, higher surgical morbidity and mortality, and stoma formation [21]. Other complications include acute appendicitis, ischemic colitis, and intussusception [22].
CT can localize an obstructing lesion with high sensitivity (96%) and specificity (93%) [22]. Left-sided malignancies are more likely to be obstructive. Obstructive lesions manifest with an intestinal caliber transition point at tumor level and upstream dilatation. A cecal lumen exceeding 12–15 cm, more likely to occur in patients with a competent ileo-cecal valve, should be an alert for imminent rupture, as should be the presence of any area of wall hypoenhancement [23].
Although perforation may occur proximal to an obstructing tumor, it more commonly occurs at the tumor site itself, due to necrosis and tissue friability [24]. It is the most lethal complication of colon cancer, with mortality rates as high as 50% due to secondary fecal peritonitis [21]. On CT, a focal defect in the bowel wall may be observed, accompanied by adjacent fat stranding, extraluminal air, and a variable amount of fluid. Perforation may be free or localized, the latter with eventual abscess formation and/or fistulation [21]. Oral contrast or contrast per rectum may help document a perforation but lack of extravasation of contrast does not rule it out, making its clinical utility questionable.
Key Point
Precise T and N staging with CT for colon cancer is challenging. However, it is highly valuable for M staging, planning curative surgery, diagnosing pre- and postoperative complications, assessing response to systemic treatment, and for long-term follow-up.
4.2.3 Evaluation of Response to Neoadjuvant Therapy in Rectal Cancer
Given the established advantage regarding local recurrence rates, standard treatment for locally advanced rectal cancer involves a combination of neoadjuvant radiation and chemotherapy, prior to total mesorectal excision (TME). It induces downsizing and downstaging of the disease in most patients, and in a variable proportion of them, 10–25% in most series, it leads to a complete response [11]. There are two main drives to re-stage rectal cancer after neoadjuvant therapy (NAT): to detect changes in the relation between the tumor and adjacent structures that may permit a less mutilating, yet still curative surgery; and to offer, in dedicated centers, the option of non-operative management to clinical complete responders [11].
4.2.3.1 Technique
Assessment of response to NAT prior to surgery relies on clinical evaluation, MR imaging and, in “Watch-and-Wait” dedicated centers, also on rectoscopy. The post-NAT MR imaging evaluation, just as in the staging setting, relies on high resolution T2-weighted images acquired in sagittal, parallel, and perpendicular planes relative to the tumor bed. For the identification of clinical complete responses, the use of diffusion-weighted imaging (DWI) may be of additional value, and given it is very sensitive to motion and air-induced susceptibility, patient preparation is determinant [25]. We recommend fasting for 6 h, a small enema 20 min before acquisition, and the administration of a spasmolytic agent in the absence of contraindications [25].
4.2.3.2 Re-staging to Plan Surgery
After NAT, the tumor may move away from anatomical landmarks or structures in a favorable manner. For instance, its inferior border may shift cranially making TME with a colo-anal anastomosis a possibility. Also, whenever a fat cushion becomes visible between the tumor bed and the mesorectal fascia at re-staging, or whenever the mesorectal fascia is reached only by very thin hypointense fibrotic spiculae, the specificity for a non-involved margin at pathology after TME may be 100% (Fig. 4.9a, b) [25]. On the other hand, whenever dense hypointense “fibrosis” reaches the mesorectal fascia, a resection beyond TME plane should be planned to achieve negative margins (Fig. 4.9c, d) [25].
Staging (a) and 11 weeks post-NAT re-staging (b) MR oblique axial T2-weighted images depicting a low rectal cancer. The tumor reached and pushed the right levator laterally at staging examination (arrow in a). After NAT, it regressed, and a thin fat plane became visible between it and the levator (arrow in b). Patient underwent abdominoperineal excision and an ypT2N0R0 specimen was obtained; another case of a very low rectal tumor invading the posterior wall of the vagina and the left levator at staging examination (arrows in c). Twelve weeks post-NAT, it regressed but a relatively large surface of contact between the hypointense fibrotic tumor bed and the posterior wall of the vagina was still apparent (arrow in d). Anteriorly extended abdominoperineal excision specimen showed a ypT3 tumor <1 mm from the anterior mesorectal fascia
It is also very important to evaluate the pelvic lymph nodes in the obturator and internal iliac compartments. Data from the Lateral Node Study Consortium found that internal iliac lymph nodes with a short axis >4 mm post-NAT were associated with a 52% likelihood of lateral local recurrence and that obturator lymph nodes with a short axis >6 mm post-NAT were associated with a higher 5-year rate of distant metastases, re-igniting the discussion on the need to remove lateral nodes surgically during TME in selected patients, which is not standard practice in western countries (Fig. 4.10) [26].
Patient with a mid-rectal cancer and an uncharacteristic 5 mm lymph node in the left iliac compartment on staging oblique axial T2-weighted MR imaging (arrow in a). Lymph node became heterogeneously hypointense after irradiation but remained 5 mm in short axis (arrow in b). At 1-year follow-up imaging, a clear lateral nodal recurrence became apparent (arrow in c). Retroperitoneal and lung metastasis were detected concomitantly
4.2.3.3 The Prognostic Value of Re-staging MR Imaging
Different assessment methods may be utilized to evaluate response of the primary tumor but the most important is the T2-weighted imaging-based magnetic resonance tumor regression grade (mrTRG) (Fig. 4.11), mrTRGs 4 and 5 being associated with worse patient survival [26].
MrTRG scale examples on oblique axial T2-weighted cropped images. MrTRG1—linear crescentic 1–2 mm endoluminal scar; mrTRG2—dense hypointense fibrosis with no obvious intermediate tumor signal intensity; mrTRG3—>50% fibrosis and visible intermediate tumor signal intensity; mrTRG4—slight response (mostly intermediate tumor signal intensity); mrTRG5—no response or tumor growth [5, 7]
Regarding low rectal cancer in particular, an mrTRG1-2 plus a tumor regression from an “unsafe” to a “safe” plane on post-NAT MR imaging is very specific for a non-involved margin at pathology (Fig. 4.12) [27].
A55-year-old female with low rectal cancer invading the internal sphincter and the left levator [red arrows in a (oblique axial) and b (coronal T2-weighted images)] who underwent NAT and was re-staged at 10 weeks. Tumor regressed from an “unsafe” to a “safe” resection plane, given a thin fat cushion became visible between the tumor bed and the levator (red arrows in c and d). An abdominoperineal excision was performed and a ypT3N0R0 resection specimen was obtained. Tumor was 3 mm away from the radial resection margin
4.2.3.4 Re-staging to Select Patients for Non-operative Management
In a variable proportion of locally advanced rectal cancer patients, there are no signs of viable tumor after NAT. In the observational studies available, the clinical criteria with the highest specificity for a pathologic complete response or a sustained clinical complete response over time are a flat white scar with or without telangiectasia at rectoscopy; and a smooth “normalized” tumor bed at digital rectal examination [28]. Regarding MR imaging, the most specific criteria depend on the analysis of T2-weighted images. They are the following:
-
an mrTRG1, corresponding either to a linear/crescentic 1–2 mm hypointense scar on the endoluminal aspect of the rectal wall or to its normalization (rarely observed in our experience) [29].
-
a positive split scar sign, corresponding to a specific layered reorganization of the rectal wall at tumor bed [30].
The lack or residual high signal intensity at high b value DWI images supports a complete response, but it is not specific.
An example of a patient with strict criteria for a complete response is provided in Fig. 4.13.
Oblique axial (a) and sagittal (b) T2-weighted images from a 68-year-old male patient with an mrT2 tumor (red arrows) involving the postero-bilateral wall at 4 cm from the anal verge. Patient was N1a (blue arrow in b) and EMVI negative. After NAT, at 11 weeks, digital rectal examination was normal, and response on MR imaging was considered mrTRG1 and split scar sign positive on axial and sagittal T2-weighted imaging (c and d). The positive high rectal lymph node regressed to 2 mm in size (blue arrow in d). No high signal intensity at tumor bed was found on DWI (e) (red arrow points to “star-shaped” normal luminal hyperintensity). At rectoscopy (f), a flat white scar with overhanging telangiectasia is observed (between arrows). These are the typical MR imaging and rectoscopy findings of a clinical complete response
Key Point
Re-staging MRI after neoadjuvant therapy in rectal cancer may document sufficient tumoral regression to support less mutilating curative surgery and help identify clinical complete responders for non-operative management in dedicated centers.
4.3 Concluding Remarks
Benign and malignant diseases of the colon and rectum include a wide spectrum of neoplastic and inflammatory disorders. Cross-sectional imaging techniques (including CT and MRI) play a crucial role in imaging of benign and malignant diseases of the colon and rectum for the primary diagnosis, risk stratification, procedural planning, treatment response evaluation, and the assessment of related extraluminal and extraintestinal pathologies and complications. Despite the significant overlap in imaging findings of different bowel conditions, understanding of leading disease patterns and specific imaging features can allow accurate diagnosis and, therefore, patients’ management.
Take-Home Messages
-
Abdominal CT and MRI are standard care for patients with colon and rectum diseases for primary diagnosis and assessment of complications. They allow accurate and reliable diagnosis of different colorectal diseases, including their localization, extension, in-depth mural involvement, enhancement pattern, and also associated pericolonic and extraintestinal findings.
-
CT is the imaging workhorse in colon cancer. It allows adequate planning of curative resection in eligible patients, pre- and postoperative diagnose of complications, distant staging, response assessment in those selected for systemic therapy and follow-up.
-
Rectal MRI plays a key role in the pre-treatment local staging of rectal cancer and enables assessment of poor prognostic factors including MRF and EMVI.
-
Re-staging of rectal cancer after neoadjuvant therapy may help select patients for less mutilating surgery or for non-operative management when an mrTRG 1/split scar positive tumor bed is observed without diffusion restriction.
References
Loftus EV. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504–17.
Panizza PSB, Viana PCC, Horvat N, et al. Inflammatory bowel disease: current role of imaging in diagnosis and detection of complications: gastrointestinal imaging. Radiographics. 2017;37(2):701–2.
Maddu KK, Mittal P, Shuaib W, Tewari A, Ibraheem O, Khosa F (2014) Colorectal emergencies and related complications: a comprehensive imaging review—imaging of colitis and complications. AJR Am J Roentgenol 203(6):1205–1216.
Kawamoto S, Horton KM, Fishman EK. Pseudomembranous colitis: spectrum of imaging findings with clinical and pathologic correlation. Radiographics. 1999;19(4):887–97.
McGettigan MJ, Menias CO, Gao ZJ, Mellnick VM, Hara AK. Imaging of drug-induced complications in the gastrointestinal system. Radiographics. 2016;36(1):71–87.
Vogel MN, Goeppert B, Maksimovic O, et al. CT features of neutropenic enterocolitis in adult patients with hematological diseases undergoing chemotherapy. Rofo. 2010;182(12):1076–81.
Capps GW, Fulcher AS, Szucs RA, Turner MA. Imaging features of radiation-induced changes in the abdomen. Radiographics. 1997;17(6):1455–73.
Brodoefel H, Bethge W, Vogel M, et al. Early and late-onset acute GvHD following hematopoietic cell transplantation: CT features of gastrointestinal involvement with clinical and pathological correlation. Eur J Radiol. 2010;73(3):594–600.
Lembcke B. Diagnosis, differential diagnoses, and classification of diverticular disease. Viszeralmedizin. 2015;31(2):95–102.
Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of rectal cancer: tumor staging, imaging techniques, and management. Radiographics. 2019;39(2):367–87.
Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2022; https://doi.org/10.1136/gutjnl-2022-327736.
Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967–76.
Haan MC de, Pickhardt PJ, Stoker J (2015) CT colonography: accuracy, acceptance, safety and position in organised population screening. Gut 64(2):342–350.
Tong G-J, Zhang G-Y, Liu J, et al. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J Clin Oncol. 2018;9(7):148–61.
Hunter C, Siddiqui M, Georgiou Delisle T, et al. CT and 3-T MRI accurately identify T3c disease in colon cancer, which strongly predicts disease-free survival. Clin Radiol. 2017;72(4):307–15.
Santiago IA, Rodrigues ER, Germano AS, et al. High-risk features in potentially resectable colon cancer: a prospective MDCT-pathology agreement study. Abdom Radiol (NY). 2016;41(10):1877–90.
Unterrainer M, Deroose CM, Herrmann K, et al. Imaging standardisation in metastatic colorectal cancer: a joint EORTC-ESOI-ESGAR expert consensus recommendation. Eur J Cancer. 2022;176:193–206.
Ko EY, Ha HK, Kim AY, et al. CT differentiation of mucinous and nonmucinous colorectal carcinoma. AJR Am J Roentgenol. 2007;188(3):785–91.
Hong EK, Landolfi F, Castagnoli F, et al. CT for lymph node staging of colon cancer: not only size but also location and number of lymph node count. Abdom Radiol (NY). 2021;46(9):4096–105.
Fernandez LM, Ibrahim RNM, Mizrahi I, DaSilva G, Wexner SD. How accurate is preoperative colonoscopic localization of colonic neoplasia? Surg Endosc. 2019;33(4):1174–9.
Yang KM, Jeong M-J, Yoon KH, Jung YT, Kwak JY. Oncologic outcome of colon cancer with perforation and obstruction. BMC Gastroenterol. 2022;22(1):247.
Kim SW, Shin HC, Kim IY, Kim YT, Kim C-J. CT findings of colonic complications associated with colon cancer. Korean J Radiol. 2010;11(2):211–21.
Herring W. Learning radiology: recognizing the basics/William Herring, MD, FACR, Vice Chairman and Residency Program Director, Albert Einstein Medical Center, Philadelphia, Pennsylvania. 3rd ed. Philadelphia, PA: Elsevier; 2016.
Taydas O, Unal E, Onur MR, Akpinar E. Role of computed tomography in intestinal obstruction. Istanbul Med J. 2018;19(2):105–12.
Santiago I, Rodrigues B, Barata M, et al. Re-staging and follow-up of rectal cancer patients with MR imaging when “Watch-and-Wait” is an option: a practical guide. Insights Imaging. 2021;12(1):114.
Schaap DP, Boogerd LSF, Konishi T, et al. Rectal cancer lateral lymph nodes: multicentre study of the impact of obturator and internal iliac nodes on oncological outcomes. Br J Surg. 2021;108(2):205–13.
Battersby NJ, How P, Moran B, et al. Prospective validation of a low rectal cancer magnetic resonance imaging staging system and development of a local recurrence risk stratification model: the MERCURY II study. Ann Surg. 2016;263(4):751–60.
van der Sande ME, Maas M, Melenhorst J, Breukink SO, van Leerdam ME, Beets GL. Predictive value of endoscopic features for a complete response after chemoradiotherapy for rectal cancer. Ann Surg. 2021;274(6):e541–7.
Jang JK, Choi SH, Park SH, et al. MR tumor regression grade for pathological complete response in rectal cancer post neoadjuvant chemoradiotherapy: a systematic review and meta-analysis for accuracy. Eur Radiol. 2020;30(4):2312–23.
Santiago I, Barata M, Figueiredo N, et al. The split scar sign as an indicator of sustained complete response after neoadjuvant therapy in rectal cancer. Eur Radiol. 2020;30(1):224–38.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Attenberger, U., Santiago, I. (2023). Benign and Malignant Diseases of the Colon and Rectum. In: Hodler, J., Kubik-Huch, R.A., Roos, J.E., von Schulthess, G.K. (eds) Diseases of the Abdomen and Pelvis 2023-2026. IDKD Springer Series. Springer, Cham. https://doi.org/10.1007/978-3-031-27355-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-27355-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-27354-4
Online ISBN: 978-3-031-27355-1
eBook Packages: MedicineMedicine (R0)