Abstract
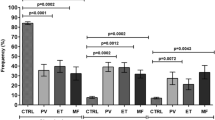
The Philadelphia-negative myeloproliferative neoplasms (MPNs), defined as clonal disorders of the hematopoietic stem cells, are characterized by the proliferation of mature myeloid cells in the bone marrow and a chronic inflammatory status impacting the initiation, progression, and symptomatology of the malignancies. There are three main entities defined as essential thrombocythemia (ET), polycythemia vera (PV), and primary myelofibrosis (PMF), and genetically classified by JAK2V617F, CALR, or MPL mutations. In MPNs, due to the overproduction of inflammatory cytokines by the neoplastic cells and non-transformed immune cells, chronic inflammation may provoke the generation and expansion of myeloid-derived suppressors cells (MDSCs) that highly influence the adaptive immune response. Although peripheral blood MDSC levels are elevated, their frequency in the bone marrow of MPNs patients is not well elucidated yet. Our results indicated increased levels of total (T)-MDSCs (CD33+HLA-DR−/low) and polymorphonuclear (PMN)-MDSCs (CD33+/HLA-DRlow/CD15+/CD14−) in the bone marrow and peripheral blood of all three types of MPNs malignancies. However, these bone marrow MDSCs-increased frequencies did not correlate with the clinical parameters, such as hepatomegaly, leukocytes, hemoglobin, or platelet levels, or with JAK2 and CALR mutations. Besides, bone marrow MDSCs, from ET, PV, and PMF patients, exhibited immunosuppressive function, determined as T-cell proliferation inhibition. Notably, the highest T-MDSCs and PMN-MDSC levels were found in PMF samples, and the increased MDSCs frequency strongly correlated with the degree of myelofibrosis. Thus, these data together indicate that the immunosuppressive MDSCs population is increased in the bone marrow of MPNs patients and may be implicated in generating a fibrotic microenvironment.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- CALR:
-
Calreticulin
- DC:
-
Dendritic cells
- ET:
-
Essential thrombocythemia
- GM-CSF:
-
Granulocyte macrophage-colony stimulating factor
- HD:
-
Healthy donors
- HSC:
-
Hematopoietic stem cell
- HU:
-
Hydroxyurea
- IL:
-
Interleukin
- JAK:
-
Janus Kinase
- LDH:
-
Lactate dehydrogenase
- MDSCs :
-
Myeloid-derived suppressor cells
- M-MDSCs:
-
Monocytic myeloid-derived suppressor cells
- MNCs:
-
Mononuclear cells
- MPL:
-
Thrombopoietin receptor
- MPNs :
-
Myeloproliferative neoplasms
- NK:
-
Natural Killer
- PMF:
-
Primary myelofibrosis
- PMN-MDSCs :
-
Polymorphonuclear myeloid-derived suppressor cells
- PV:
-
Polycythemia vera
- SE:
-
Sedimentation
- STAT:
-
Signal transducer and activators of transcription
- TGF-β1 :
-
Transforming growth factor-β1
- T-MDSCS:
-
Total myeloid-derived suppressors cells
- TNF-α:
-
Tumor necrosis factor-α
- WBC:
-
White blood cells
- WHO:
-
World Health Organization
References
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF et al. (2022 Jul) The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia 36(7):1703–1719. https://doi.org/10.1038/s41375-022-01613-1. Epub 2022 Jun 22. PMID: 35732831; PMCID: PMC9252913
Nasillo V, Riva G, Paolini A, Forghieri F, Roncati L, Lusenti B et al (2021) Inflammatory microenvironment and specific T cells in myeloproliferative neoplasms: immunopathogenesis and novel immunotherapies. Int J Mol Sci 22(4):1906. https://doi.org/10.3390/ijms22041906.PMID:33672997;PMCID:PMC7918142
Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L et al. (2014 Oct 16) Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 124(16):2507–13; quiz 2615. https://doi.org/10.1182/blood-2014-05-579136. Epub 2014 Jul 18. PMID: 25037629; PMCID: PMC4199952
Geyer HL, Dueck AC, Scherber RM, Mesa RA (2015) Impact of inflammation on myeloproliferative neoplasm symptom development. Med Inflamm 2015:284706. https://doi.org/10.1155/2015/284706. Epub 2015 Oct 11. PMID: 26538823; PMCID: PMC4619953
Mendez Luque LF, Blackmon AL, Ramanathan G, Fleischman AG (2019 Jun) Key role of inflammation in myeloproliferative neoplasms: instigator of disease initiation, progression and symptoms. Curr Hematol Malig Rep 14(3):145–153. https://doi.org/10.1007/s11899-019-00508-w. PMID: 31119475; PMCID: PMC7746200
Wang Y, Zuo X (2019) Cytokines frequently implicated in myeloproliferative neoplasms. Cytokine X 1(1):100005. https://doi.org/10.1016/j.cytox.2019.100005.PMID:33604548;PMCID:PMC7885877
Bhuria V, Baldauf CK, Schraven B, Fischer T (2022) Thromboinflammation in Myeloproliferative Neoplasms (MPN)-a puzzle still to be solved. Int J Mol Sci 23(6):3206. https://doi.org/10.3390/ijms23063206.PMID:35328626;PMCID:PMC8954909
Hermouet S, Bigot-Corbel E, Gardie B (2015) Pathogenesis of myeloproliferative neoplasms: role and mechanisms of chronic inflammation. Med Inflamm 2015:145293. https://doi.org/10.1155/2015/145293. Epub 2015 Oct 11. PMID: 26538820; PMCID: PMC4619950
Zahr AA, Salama ME, Carreau N, Tremblay D, Verstovsek S, Mesa R, Hoffman R, Mascarenhas J (2016) Bone marrow fibrosis in myelofibrosis: pathogenesis, prognosis and targeted strategies. Haematologica 101(6):660–671. https://doi.org/10.3324/haematol.2015.141283.PMID:27252511;PMCID:PMC5013940
Vukotić M, Kapor S, Dragojević T, Đikić D, Mitrović Ajtić O, Diklić M et al. (2022 Mar) Inhibition of proinflammatory signaling impairs fibrosis of bone marrow mesenchymal stromal cells in myeloproliferative neoplasms. Exp Mol Med 54(3):273–284. https://doi.org/10.1038/s12276-022-00742-y. Epub 2022 Mar 14. PMID: 35288649; PMCID: PMC8980093
Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ et al (2005) Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 7(4):387–397. https://doi.org/10.1016/j.ccr.2005.03.023. PMID: 15837627
Wolf A, Eulenfeld R, Gäbler K, Rolvering C, Haan S, Behrmann I et al. (2013 Jul 1) JAK2-V617F-induced MAPK activity is regulated by PI3K and acts synergistically with PI3K on the proliferation of JAK2-V617F-positive cells. JAKSTAT. 2(3):e24574. https://doi.org/10.4161/jkst.24574. Epub 2013 Apr 8. PMID: 24069558; PMCID: PMC3772110
Hu X, Li J, Fu M, Zhao X, Wang W (2021) The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 6(1):402. https://doi.org/10.1038/s41392-021-00791-1.PMID:34824210;PMCID:PMC8617206
Edahiro Y, Araki M, Komatsu N (2020 Aug) Mechanism underlying the development of myeloproliferative neoplasms through mutant calreticulin. Cancer Sci 111(8):2682–2688. https://doi.org/10.1111/cas.14503. Epub 2020 Jun 27. PMID: 32462673; PMCID: PMC7419020
Masselli E, Pozzi G, Gobbi G, Merighi S, Gessi S, Vitale M, Carubbi C (2020) Cytokine profiling in myeloproliferative neoplasms: overview on phenotype correlation, outcome prediction, and role of genetic variants. Cells 9(9):2136. https://doi.org/10.3390/cells9092136.PMID:32967342;PMCID:PMC7564952
Mojsilovic S, Mojsilovic SS, Bjelica S, Santibanez JF (2022 Jan) Transforming growth factor-beta1 and myeloid-derived suppressor cells: A cancerous partnership. Dev Dyn 251(1):105-124. https://doi.org/10.1002/dvdy.339. Epub 2021 Apr 8. PMID: 33797140
Wang S, Tan Q, Hou Y, Dou H (2021) Emerging roles of myeloid-derived suppressor cells in diabetes. Front Pharmacol 16(12):798320. https://doi.org/10.3389/fphar.2021.798320.PMID:34975496;PMCID:PMC8716856
Medina E, Hartl D (2018) Myeloid-Derived Suppressor Cells in Infection: A General Overview. J Innate Immun 10(5–6):407–413. https://doi.org/10.1159/000489830. Epub 2018 Jun 26. PMID: 29945134; PMCID: PMC6784037
Veglia F, Perego M, Gabrilovich D (2018 Feb) Myeloid-derived suppressor cells coming of age. Nat Immunol 19(2):108–119. https://doi.org/10.1038/s41590-017-0022-x. Epub 2018 Jan 18. PMID: 29348500; PMCID: PMC5854158
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF et al (2016) Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 6(7):12150. https://doi.org/10.1038/ncomms12150.PMID:27381735;PMCID:PMC4935811
Bizymi N, Bjelica S, Kittang AO, Mojsilovic S, Velegraki M, Pontikoglou C et al (2019) Myeloid-derived suppressor cells in hematologic diseases: promising biomarkers and treatment targets. Hemasphere 3(1):e168. https://doi.org/10.1097/HS9.0000000000000168.PMID:31723807;PMCID:PMC6745940
Veglia F, Sanseviero E, Gabrilovich DI (2021 Aug) Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol 21(8):485–498. https://doi.org/10.1038/s41577-020-00490-y. Epub 2021 Feb 1. PMID: 33526920; PMCID: PMC7849958
Gondois-Rey F, Paul M, Alcaraz F, Bourass S, Monnier J, Malissen N et al (2021) Identification of an immature subset of PMN-MDSC correlated to response to checkpoint inhibitor therapy in patients with metastatic melanoma. Cancers (Basel) 13(6):1362. https://doi.org/10.3390/cancers13061362.PMID:33802925;PMCID:PMC8002694
Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moreno C, Scumpia PO, Laface DM et al. (2011 Mar–Apr) A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Mol Med 17(3–4):281–92. https://doi.org/10.2119/molmed.2010.00178. Epub 2010 Nov 12. PMID: 21085745; PMCID: PMC3060988.
Sica A, Guarneri V, Gennari A (2019) Myelopoiesis, metabolism and therapy: a crucial crossroads in cancer progression. Cell Stress 3(9):284–294. https://doi.org/10.15698/cst2019.09.197.PMID:31535085;PMCID:PMC6732213
Kumar V, Patel S, Tcyganov E, Gabrilovich DI (2016 Mar) The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol 37(3):208–220. https://doi.org/10.1016/j.it.2016.01.004. Epub 2016 Feb 6. PMID: 26858199; PMCID: PMC4775398
Gabrilovich DI (2017) Myeloid-derived suppressor cells. Cancer Immunol Res 5(1):3–8. https://doi.org/10.1158/2326-6066.CIR-16-0297.PMID:28052991;PMCID:PMC5426480
Kalathil SG, Thanavala Y (2021 Mar) Importance of myeloid derived suppressor cells in cancer from a biomarker perspective. Cell Immunol 361:104280. https://doi.org/10.1016/j.cellimm.2020.104280. Epub 2020 Dec 31. PMID: 33445053; PMCID: PMC9204650
Coşkun Bİ (2021) The osteocyte as a director of bone metabolism. Arch Rheumatol. 36(4):617–619. https://doi.org/10.46497/ArchRheumatol.2021.8632.PMID:35382360;PMCID:PMC8957769
Zanetti C, Krause DS (2020 Sep) Caught in the net: the extracellular matrix of the bone marrow in normal hematopoiesis and leukemia. Exp Hematol 89:13–25. https://doi.org/10.1016/j.exphem.2020.07.010. Epub 2020 Aug 2. PMID: 32755619
Spampinato M, Giallongo C, Romano A, Longhitano L, La Spina E, Avola R et al (2021) Focus on osteosclerotic progression in primary myelofibrosis. Biomolecules 11(1):122. https://doi.org/10.3390/biom11010122.PMID:33477816;PMCID:PMC7832894
Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A (2005) European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica 90(8):1128–1132 PMID: 16079113
Yao JC, Oetjen KA, Wang T, Xu H, Abou-Ezzi G, Krambs JR, Uttarwar S, Duncavage EJ, Link DC (2022) TGF-β signaling in myeloproliferative neoplasms contributes to myelofibrosis without disrupting the hematopoietic niche. J Clin Invest 132(11):e154092. https://doi.org/10.1172/JCI154092.PMID:35439167;PMCID:PMC9151699
Verstovsek S, Manshouri T, Pilling D, Bueso-Ramos CE, Newberry KJ, Prijic S et al. (2016 Aug 22) Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med 213(9):1723–40. https://doi.org/10.1084/jem.20160283. Epub 2016 Aug 1. PMID: 27481130; PMCID: PMC4995084
Casacuberta-Serra S, Parés M, Golbano A, Coves E, Espejo C, Barquinero J (2017 Jul) Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes. Immunol Cell Biol 95(6):538–548. https://doi.org/10.1038/icb.2017.4. Epub 2017 Jan 21. PMID: 28108746
Wang Y, Ma Y, Fang Y, Wu S, Liu L, Fu D, Shen X (2012) Regulatory T cell: a protection for tumour cells. J Cell Mol Med 16(3):425–436. https://doi.org/10.1111/j.1582-4934.2011.01437.x.PMID:21895966;PMCID:PMC3822920
Wang JC, Kundra A, Andrei M, Baptiste S, Chen C, Wong C, Sindhu H (2016) Myeloid-derived suppressor cells in patients with myeloproliferative neoplasm. Leuk Res 43:39–43. https://doi.org/10.1016/j.leukres.2016.02.004. Epub 2016 Feb 16 PMID: 26943702
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P et al (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 15(6):2148–2157. https://doi.org/10.1158/1078-0432.CCR-08-1332. Epub 2009 Mar 10 PMID: 19276286
Kapor S, Vukotić M, Subotički T, Đikić D, Mitrović Ajtić O, Radojković M et al (2021) Hydroxyurea induces bone marrow mesenchymal stromal cells senescence and modifies cell functionality in vitro. J Pers Med 11(11):1048. https://doi.org/10.3390/jpm11111048.PMID:34834400;PMCID:PMC8619969
Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P et al (2018) The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J 8(2):15. https://doi.org/10.1038/s41408-018-0054-y.PMID:29426921;PMCID:PMC5807384
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405. https://doi.org/10.1182/blood-2016-03-643544. Epub 2016 Apr 11 PMID: 27069254
Nazha A, Khoury JD, Rampal RK, Daver N (2015 Oct) Fibrogenesis in primary myelofibrosis: diagnostic, clinical, and therapeutic implications. Oncologist 20(10):1154–1160. https://doi.org/10.1634/theoncologist.2015-0094. Epub 2015 Aug 24. PMID: 26304912; PMCID: PMC4591957
Kreipe H, Büsche G, Bock O, Hussein K (2012) Myelofibrosis: molecular and cell biological aspects. Fibrogenesis Tissue Repair 5(Suppl 1):S21. https://doi.org/10.1186/1755-1536-5-S1-S21.PMID:23259436;PMCID:PMC3368793
Wu Y, Yi M, Niu M, Mei Q, Wu K (2022) Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer 21(1):184. https://doi.org/10.1186/s12943-022-01657-y.PMID:36163047;PMCID:PMC9513992
Gunes EG, Rosen ST, Querfeld C (2020) The role of myeloid-derived suppressor cells in hematologic malignancies. Curr Opin Oncol 32(5):518–526. https://doi.org/10.1097/CCO.0000000000000662.PMID:32675593;PMCID:PMC7735379
Barosi G (2014) An immune dysregulation in MPN. Curr Hematol Malig Rep 9(4):331–339. https://doi.org/10.1007/s11899-014-0227-0. PMID: 25139710
Tumino N, Di Pace AL, Besi F, Quatrini L, Vacca P, Moretta L (2021) Interaction between MDSC and NK cells in solid and hematological malignancies: impact on HSCT. Front Immunol 12(12):638841. https://doi.org/10.3389/fimmu.2021.638841.PMID:33679798;PMCID:PMC7928402
García-Gutiérrez V, Hernández-Boluda JC (2019) Tyrosine kinase inhibitors available for chronic myeloid leukemia: efficacy and safety. Front Oncol 3(9):603. https://doi.org/10.3389/fonc.2019.00603.PMID:31334123;PMCID:PMC6617580
Christiansson L, Söderlund S, Mangsbo S, Hjorth-Hansen H, Höglund M, Markevärn B et al (2015) The tyrosine kinase inhibitors imatinib and dasatinib reduce myeloid suppressor cells and release effector lymphocyte responses. Mol Cancer Ther 14(5):1181–1191. https://doi.org/10.1158/1535-7163.MCT-14-0849. Epub 2015 Mar 11 PMID: 25761894
Loscocco GG, Vannucchi AM (2022) Role of JAK inhibitors in myeloproliferative neoplasms: current point of view and perspectives. Int J Hematol 115(5):626–644. https://doi.org/10.1007/s12185-022-03335-7. Epub 2022 Mar 29 PMID: 35352288
Cuthbert D, Stein BL (2019) Therapy-associated leukemic transformation in myeloproliferative neoplasms—What do we know? Best Pract Res Clin Haematol 32(1):65–73. https://doi.org/10.1016/j.beha.2019.02.004. Epub 2019 Feb 8 PMID: 30927977
Kapor S, Santibanez JF (2021) Myeloid-derived suppressor cells and mesenchymal stem/stromal cells in myeloid malignancies. J Clin Med 10(13):2788. https://doi.org/10.3390/jcm10132788.PMID:34202907;PMCID:PMC8268878
Hyun SY, Na EJ, Jang JE, Chung H, Kim SJ, Kim JS, Kong JH, Shim KY, Lee JI, Min YH, Cheong JW (2020 Oct) Immunosuppressive role of CD11b+ CD33+ HLA-DR- myeloid-derived suppressor cells-like blast subpopulation in acute myeloid leukemia. Cancer Med 9(19):7007–7017. https://doi.org/10.1002/cam4.3360. Epub 2020 Aug 11. PMID: 32780544; PMCID: PMC7541151
Stubbins RJ, Platzbecker U, Karsan A (2022) Inflammation and myeloid malignancy: quenching the flame. Blood 140(10):1067–1074. https://doi.org/10.1182/blood.2021015162. PMID: 35468199
Kleppe M, Kwak M, Koppikar P, Riester M, Keller M, Bastian L et al. (2015 Mar) JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov 5(3):316–31. https://doi.org/10.1158/2159-8290.CD-14-0736. Epub 2015 Jan 8. PMID: 25572172; PMCID: PMC4355105
Giallongo C, Parrinello N, Brundo MV, Raccuia SA, Di Rosa M, La Cava P, Tibullo D (2015) Myeloid derived suppressor cells in chronic myeloid leukemia. Front Oncol 15(5):107. https://doi.org/10.3389/fonc.2015.00107.PMID:26029664;PMCID:PMC4432672
Sabattini E, Pizzi M, Agostinelli C, Bertuzzi C, Sagramoso Sacchetti CA, Palandri F, Gianelli U (2021) Progression in Ph-chromosome-negative myeloproliferative neoplasms: an overview on pathologic issues and molecular determinants. Cancers (Basel) 13(21):5531. https://doi.org/10.3390/cancers13215531.PMID:34771693;PMCID:PMC8583143
Thiele J, Kvasnicka HM, Orazi A, Tefferi A, Birgegard G, Barbui T et al. (2017) WHO classification of tumours of haematopoietic and lymphoid tissues. IARC Geneva, Switzerland, pp 39–50
Spivak JL (2017) Myeloproliferative neoplasms. N Engl J Med 377(9):895–896. https://doi.org/10.1056/NEJMc1708485. PMID: 28854086
Mannelli F (2021) Acute myeloid leukemia evolving from myeloproliferative neoplasms: many sides of a challenging disease. J Clin Med 10(3):436. https://doi.org/10.3390/jcm10030436.PMID:33498691;PMCID:PMC7866045
Chagraoui H, Wendling F, Vainchenker W (2006) Pathogenesis of myelofibrosis with myeloid metaplasia: insight from mouse models. Best Pract Res Clin Haematol 19(3):399–412. https://doi.org/10.1016/j.beha.2005.07.002. PMID: 16781480
Palumbo GA, Parrinello NL, Giallongo C, D’Amico E, Zanghì A, Puglisi F et al (2019) Monocytic myeloid derived suppressor cells in hematological malignancies. Int J Mol Sci 20(21):5459. https://doi.org/10.3390/ijms20215459.PMID:31683978;PMCID:PMC6862591
Yao JC, Oetjen KA, Wang T, Xu H, Abou-Ezzi G, Krambs JR et al (2022) TGF-β signaling in myeloproliferative neoplasms contributes to myelofibrosis without disrupting the hematopoietic niche. J Clin Invest 132(11):e154092. https://doi.org/10.1172/JCI154092.PMID:35439167;PMCID:PMC9151699
Agarwal A, Morrone K, Bartenstein M, Zhao ZJ, Verma A, Goel S (2016) Bone marrow fibrosis in primary myelofibrosis: pathogenic mechanisms and the role of TGF-β. Stem Cell Investig 26(3):5. https://doi.org/10.3978/j.issn.2306-9759.2016.02.03.PMID:27358897;PMCID:PMC4923632
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12(6):325–338. https://doi.org/10.1038/nrneph.2016.48. Epub 2016 Apr 25 PMID: 27108839
Dong M, Blobe GC (2006 Jun 15) Role of transforming growth factor-beta in hematologic malignancies. Blood 107(12):4589–96. https://doi.org/10.1182/blood-2005-10-4169. Epub 2006 Feb 16. PMID: 16484590; PMCID: PMC1895802
Deng X, Li X, Guo X, Lu Y, Xie Y, Huang X et al. (2022 Jun) Myeloid-derived suppressor cells promote tumor growth and sorafenib resistance by inducing FGF1 upregulation and fibrosis. Neoplasia 28:100788. https://doi.org/10.1016/j.neo.2022.100788. Epub 2022 Apr 1. PMID: 35378464; PMCID: PMC8980488
Qiu Y, Cao Y, Tu G, Li J, Su Y, Fang F et al (2021) Myeloid-derived suppressor cells alleviate renal fibrosis progression via regulation of CCL5-CCR5 axis. Front Immunol 10(12):698894. https://doi.org/10.3389/fimmu.2021.698894
Statements and Declarations
Funding: This study was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant number 451-03-9/2021-14/200015).
Disclosure of Interests: All authors declare they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Approval was granted by the ethics committee of the Clinical Center of Serbia, Belgrade (decision number 4/1). This article does not contain any studies with animals performed by any of the authors.
Data Availability Statement: The material supporting the conclusions of this study have been included within the article
Acknowledgements: We thank the support of the visiting professor program of UBO to JFS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kapor, S. et al. (2023). Increase in Frequency of Myeloid-Derived Suppressor Cells in the Bone Marrow of Myeloproliferative Neoplasm: Potential Implications in Myelofibrosis. In: Simon, F., Bernabeu, C. (eds) Advances in Molecular Pathology. Advances in Experimental Medicine and Biology, vol 1408. Springer, Cham. https://doi.org/10.1007/978-3-031-26163-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-26163-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-26162-6
Online ISBN: 978-3-031-26163-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)