Abstract
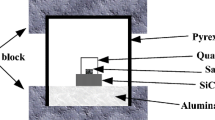
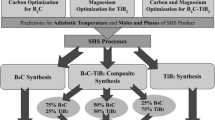
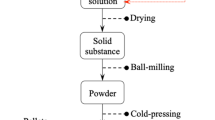
In this study, B4C-TiB2 nanocomposite powder was synthesized from oxide raw materials with the principle of magnesiothermic reduction in B2O3–TiO2–Mg–C system by SHS method. For the SHS process, Mg and C stoichiometries were optimized with thermochemical simulation, and composite charge stoichiometry and Mg particle size were optimized with XRD, BET and SEM analyzes. Optimization of acid concentration, leaching temperature, and leaching time parameters has been provided for the HCl leaching processes carried out to remove undesired by-products after SHS. In addition, pH and temperature changes during leaching were analyzed and an innovative application of modified leaching with H2O2 and carbonic acid addition was investigated. The results showed that by optimizing the process steps for the synthesis of B4C–TiB2 composite nanoparticle by the SHS method, a commercial grade product with a surface area of 30.6 m2/g, and a particle size of 193 nm was obtained.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dariel MP, Frage N (2012) Reaction bonded boron carbide: recent developments. Adv Appl Ceram 111(5–6):301–310. https://doi.org/10.1179/1743676111Y.0000000078
Xin L, Minjun L, Shuaibo G, Shu Y, Xiaofeng W, Pengfei X (2019) Effect of initial compositions on boron carbide synthesis and corresponding growth mechanism. Adv Appl Ceram 118(8):442–450. https://doi.org/10.1080/17436753.2019.1664792
Yaşar ZA, Haber RA (2021) Effect of sintering temperature and applied pressure on the properties of boron carbide-silicon carbide composites. J Superhard Mater 43:392–404. https://doi.org/10.3103/S1063457621060022
Crouch IG, Eu B (2017) Ballistic testing methodologies, vol. 11 of the science of armour materials. Woodhead Publishing, pp 639–673. https://doi.org/10.1016/B978-0-08-100704-4.00011-6
Domnich V, Reynaud S, Haber R, Chhowalla M (2011) Boron carbide: structure, properties and stability under stress. J Am Ceram Soc 64(11):3605–3628. https://doi.org/10.1111/j.1551-2916.2011.04865.x
Savio SG, Sambasiva Rao A, Rama Subba Reddy P, Madhu V (2019) Microstructure and ballistic performance of hot pressed and reaction bonded boron carbides against an armour piercing projectile. Adv Appl Ceram 118(5):264–273. https://doi.org/10.1080/17436753.2018.1564416
Wood C (1988) Materials for thermoelectric energy conversion. Rep Prog Phys 51(4):459. https://doi.org/10.1088/0034-4885/51/4/001
Suri AK, Subramanian C, Sonber JK, Ch MTSR (2010) Synthesis and consolidation of boron carbide: a review. Int Mater Rev 55(1):4–40. https://doi.org/10.1179/095066009X12506721665211
Sauerschnig P, Watts JL, Vaney JB, Talbot PC, Alarco JA, Mackinnon IDR et al (2020) Thermoelectric properties of phase pure boron carbide prepared by a solution-based method. Adv Appl Ceram 119(2):97–106. https://doi.org/10.1080/17436753.2019.1705017
Mukhopadhyay A, Raju GB, Basu B, Suri AK (2009) Correlation between phase evolution, mechanical properties and instrumented indentation response of TiB2-based ceramics. J Euro Ceramics Soc 29(3):505–516. https://doi.org/10.1016/j.jeurceramsoc.2008.06.030
Ricceri R, Matteazzi P (2004) A fast and low-cost room temperature process for TiB2 formation by mechanosynthesis. Mater Sci Eng, A 379(1–2):341–346. https://doi.org/10.1016/j.msea.2004.02.064
Górnya G, Rączkaa M, Stobierskia L, Rożniatowskib K, Rutkowskia P (2009) Ceramic composite Ti3SiC2–TiB2-Microstructure and mechanical properties. Mater Charact 60(10):1168–1174. https://doi.org/10.1016/j.matchar.2009.03.011
Bilgi E, Camurlu HE, Akgun B, Topkaya Y, Sevinc N (2008) Formation of TiB2 by volume combustion and mechanochemical process. Mater Res Bull 43(4):873–881. https://doi.org/10.1016/j.materresbull.2007.05.001
Madhav Reddy K, Guo JJ, Shinoda Y, Fujita T, Hirata A, Singh JP et al (2012) Enhanced mechanical properties of nanocrystalline boron carbide by nanoporosity and interface phases. Nat Commun 3:1052. https://doi.org/10.1038/ncomms2047
Heydari MS, Baharvandi HR (2015) Comparing the effects of different sintering methods for ceramics on the physical and mechanical properties of B4C–TiB2 nanocomposites. Int J Refract Metal Hard Mater 51:224–232. https://doi.org/10.1016/j.ijrmhm.2015.04.003
Ivzhenko VV, Kryl’ AO, Kryl’ YA et al (2014) Study of aeroabrasive wear of hot-pressed materials of the B4C–TiB2 system. J Superhard Mater 36:187–192. https://doi.org/10.3103/S106345761403006X
Munir ZA, Anselmi-Tamburini U (1989) Self-propagating exothermic reactions: the synthesis of high-temperature materials by combustion. Mater Sci Rep 3(7–8):277–365. https://doi.org/10.1016/0920-2307(89)90001-7
Xue H, Munir ZA (1996) Extending the compositional limit of combustion synthesized B4C–TiB2 composites by field activation. Metall Mater Trans B 27:475–480
Fard HSP, Baharvandi HR, Abdizadeh H, Shahbahrami B (2008) Chemical synthesis of nano-titanium diboride powders by borothermic reduction. Int J Mod Phys B 22(18–19):3179–3184. https://doi.org/10.1142/S0217979208048085
Pei LZ, Xiao HN (2009) B4C–TiB2 composite powders prepared by carbothermal reduction method. J Mater Process Technol 209(4):2122–2127. https://doi.org/10.1016/j.jmatprotec.2008.05.003
Cakir E, Ergun C, Sahin FC, Erden I (2010) In situ synthesis of B4C/TiB2 composites from low cost sugar based precursor. Defect Diffusion Forum 297–301:52–56. https://doi.org/10.4028/www.scientific.net/DDF.297-301.52
Hongqiang R, Haifei X, Liang Y, Peng L, Xiaodong L, Guanming Q (2007) Microstructure of TiB2/B4C composites with 1% Y2O3 prepared by co-precipitating and in situ synthesis. J Rare Earths 25(1):42–45. https://doi.org/10.1016/S1002-0721(07)60520-1
Biedunkiewicz A, Figiel P, Gabriel U, Sabara M, Lenart S (2011) Synthesis and characteristics of nanocrystalline materials in Ti, B, C and N containing system. Central Euro J Phys 9(2):417–422. https://doi.org/10.2478/s11534-010-0121-x
Nikzad L, Licheri R, Vaezi MR, Orrù R, Cao G (2012) Chemically and mechanically activated combustion synthesis of B4C–TiB2 composites. Int J Refract Metal Hard Mater 35:41–48. https://doi.org/10.1016/j.ijrmhm.2012.04.001
Shojaie Bahaabad M (2017) Mechanically activated combustion synthesis of B4C–TiB2 nanocomposite powder. J Adv Mater Process 5(1):13–21
Azatyan TS, Mal’stev VM, Merzhanov AG, Seleznev VA (1977) Spectral-optical investigation of the mechanism of the combustion of mixtures of titanium and carbon. Combust, Explos, Shock Waves 13:156–158
Fan QC, Chai HF, Jin ZH (2002) Effects of particle size of reactant on characteristics of combustion synthesis of TiC-Fe cermet. J Mater Sci 37:2251–2257
Zhang EL, Zeng SY, Yang B, Li QC, Ma MZ (1999) A study on the kinetic process of reaction synthesis of TiC: Part I. Exp Res Theor Model, Metall Mater Trans A 30:1147–1151
Yang YF, Wang HY, Zhao RY, Liang YH, Zhan L, Jiang QC (2008) Effects of C particle size on the ignition and combustion characteristics of the SHS reaction in the 20 wt.% Ni–Ti–C system. J Alloy Compd 460:276–282
Alkan M, Sonmez MS, Derin B, Yucel O (2012) Effect of initial composition on boron carbide production by SHS process followed by acid leaching. Solid State Sci. https://doi.org/10.1016/j.solidstatesciences.2012.07.004
Turan A, Bugdayci M, Yucel O (2015) Self-propagating high temperature synthesis of TiB2. High Temp Mater Proc https://doi.org/10.1515/htmp-2014-0021
İpekci M, Acar S, Elmadağlı M, Hennicke J, Balcı Ö, Somer M (2017) Production of TiB2 by SHS and HCl leaching at different temperatures: Characterization and investigation of sintering behavior by SPS. Ceram Int 43(2):2039–2045. https://doi.org/10.1016/j.ceramint.2016.10.174
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Coban, O., Bugdayci, M., Baslayici, S., Acma, M.E. (2023). Combustion Synthesis of B4C–TiB2 Composite Nanoparticle by Self-Propagating High-Temperature Synthesis (SHS) in B2O3–TiO2–Mg–C System. In: Li, B., et al. Advances in Powder and Ceramic Materials Science 2023. TMS 2023. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-22622-9_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-22622-9_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22621-2
Online ISBN: 978-3-031-22622-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)