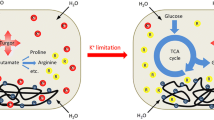
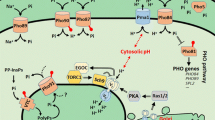
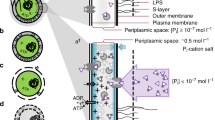
Abstract
The majority of cellular phosphate (PO4-3; Pi) exists as nucleoside triphosphates, mainly adenosine triphosphate (ATP), and ribosomal RNA (rRNA). ATP and rRNA are also the largest cytoplasmic reservoirs of magnesium (Mg2+), the most abundant divalent cation in living cells. The co-occurrence of these ionic species in the cytoplasm is not coincidental. Decades of work in the Pi and Mg2+ starvation responses of two model enteric bacteria, Escherichia coli and Salmonella enterica, have led to the realization that the metabolisms of Pi and Mg2+ are interconnected. Bacteria must acquire these nutrients in a coordinated manner to achieve balanced growth and avoid loss of viability. In this chapter, we will review how bacteria sense and respond to fluctuations in environmental and intracellular Pi and Mg2+ levels. We will also discuss how these two compounds are functionally linked, and how cells elicit physiological responses to maintain their homeostasis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Alix E, Blanc-Potard AB (2008) Peptide-assisted degradation of the Salmonella MgtC virulence factor. EMBO J 27(3):546–557
Almeida LG De, Ortiz H, Schneider RP, Spira B (2015) phoU inactivation in Pseudomonas aeruginosa enhances accumulation of ppGpp and polyphosphate. Appl Environ Microbiol 81(9):3006–3015
Bachhawat P, Swapna GVT, Montelione GT, Stock AM (2005) Mechanism of activation for transcription factor PhoB suggested by different modes of dimerization in the inactive and active states. Structure 13(9):1353–1363
Baek JH, Lee SY (2006) Novel gene members in the Pho regulon of Escherichia coli. FEMS Microbiol Lett 264(1):104–109
Beard SJ, Hashim R, Wu G, Binet MR, Hughes MN, Poole RK (2000) Evidence for the transport of zinc(II) ions via the Pit inorganic phosphate transport system in Escherichia coli. FEMS Microbiol Lett 184(2):231–235
Bennett RL, Malamy MH (1970) Arsenate resistant mutants of Escherichia coli and phosphate transport. Biochem Biophys Res Commun 40(2):496–503
Biber J, Custer M, Magagnin S, Hayes G, Werner A, Lötscher M et al (1996) Renal Na/Pi-cotransporters. Kidney Int 49(4):981–985
Bishop RE, Gibbons HS, Guina T et al (2000) Transfer of palmitate from phospholipids to lipid A in outer membranes of Gram-negative bacteria. EMBO J 19(19):5071–5080
Blanco AG, Canals A, Bernués J, Solà M, Coll M (2011) The structure of a transcription activation subcomplex reveals how σ70 is recruited to PhoB promoters. EMBO J 30(18):3776–3785
Botella E, Devine SK, Hubner S, Salzberg LI, Gale RT, Brown ED et al (2014) PhoR autokinase activity is controlled by an intermediate in wall teichoic acid metabolism that is sensed by the intracellular PAS domain during the PhoPR-mediated phosphate limitation response of Bacillus subtilis. Mol Microbiol 94(6):1242–1259
Botella E, Hübner S, Hokamp K, Hansen A, Bisicchia P, Noone D et al (2011) Cell envelope gene expression in phosphate-limited Bacillus subtilis cells. Microbiology 157(Pt 9):2470–2484
Bremer H, Dennis PP (2008) Modulation of chemical composition and other parameters of the cell at different exponential growth rates. EcoSal Plus 3(1)
Browning DF, Busby SJW (2004) The regulation of bacterial transcription initiation. Nat Rev Microbiol 2(1):57–65
Bruckner R, Titgemeyer F (2002) Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett 209(2):141–148
Bruna RE, Kendra CG, Groisman EA, Pontes MH (2021) Limitation of phosphate assimilation maintains cytoplasmic magnesium homeostasis. Proc Natl Acad Sci U S A 118(11):e2021370118
Carmany DO, Hollingsworth K, McCleary WR (2003) Genetic and biochemical studies of phosphatase activity of PhoR. J Bacteriol 185(3):1112–1115
Castelli ME, García Véscovi E, Soncini FC (2000) The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J Biol Chem 275(30):22948–22954
Chamnongpol S, Cromie M, Groisman EA (2003) Mg2+ sensing by the Mg2+ sensor PhoQ of Salmonella enterica. J Mol Biol 325(4):795–807
Chen HD, Groisman EA (2013) The biology of the PmrA/PmrB two-component system: the major regulator of lipopolysaccharide modifications. Annu Rev Microbiol 67:83–112
Cho US, Bader MW, Amaya MF, Daley ME, Klevit RE, Miller SI et al (2006) Metal bridges between the PhoQ sensor domain and the membrane regulate transmembrane signaling. J Mol Biol 356(5):1193–1206
Choi E, Lee KY, Shin D (2012) The MgtR regulatory peptide negatively controls expression of the MgtA Mg2+ transporter in Salmonella enterica serovar Typhimurium. Biochem Biophys Res Commun 417(1):318–323
Christian Perez J, Groisman EA (2009) Transcription factor function and promoter architecture govern the evolution of bacterial regulons. Proc Natl Acad Sci U S A 106(11):4319–4324
Christian Perez J, Latifi T, Groisman EA (2008) Overcoming H-NS-mediated transcriptional silencing of horizontally acquired genes by the PhoP and SlyA proteins in Salmonella enterica. J Biol Chem 283(16):10773–10783
Cox GB, Webb D, Godovac-Zimmermann J, Rosenberg H (1988) Arg-220 of the PstA protein is required for phosphate transport through the phosphate-specific transport system in Escherichia coli but not for alkaline phosphatase repression. J Bacteriol 170(5):2283–2286
Cox GB, Webb D, Rosenberg H (1989) Specific amino acid residues in both the PstB and PstC proteins are required for phosphate transport by the Escherichia coli Pst system. J Bacteriol 171(3):1531–1534
Cromie MJ, Shi Y, Latifi T, Groisman EA (2006) An RNA sensor for intracellular Mg2+. Cell 125(1):71–84
Danhorn T, Hentzer M, Givskov M, Parsek MR, Fuqua C (2004) Phosphorus limitation enhances biofilm formation of the plant pathogen Agrobacterium tumefaciens through the PhoR-PhoB regulatory system. J Bacteriol 186(14):4492–4501
diCenzo GC, Sharthiya H, Nanda A, Zamani M, Finan TM (2017) PhoU allows rapid adaptation to high phosphate concentrations by modulating PstSCAB transport rate in Sinorhizobium meliloti. J Bacteriol 199(18):e00143–e00117
Egan SE, Fliege R, Tong S, Shibata A, Wolf RE Jr, Conway T (1992) Molecular characterization of the Entner-Doudoroff pathway in Escherichia coli: sequence analysis and localization of promoters for the edd-eda operon. J Bacteriol 174(14):4638–4646
Ellison DW, McCleary WR (2000) The unphosphorylated receiver domain of PhoB silences the activity of its output domain. J Bacteriol 182(23):6592–6597
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T et al (2003) Growth rate-stoichiometry couplings in diverse biota. Ecol Lett 6(10):936–943
Feuerstein BG, Williams LD, Basu HS, Marton LJ (1991) Implications and concepts of polyamine-nucleic acid interactions. J Cell Biochem 46(1):37–47
Filloux A, Bally M, Soscia C, Murgier M, Lazdunski A (1988) Phosphate regulation in Pseudomonas aeruginosa: cloning of the alkaline phosphatase gene and identification of phoB- and phoR-like genes. Mol Gen Genet 212(3):510–513
Foster AW, Osman D, Robinson NJ (2014) Metal preferences and metallation. J Biol Chem 289(41):28095–28103
Gao R, Stock AM (2015) Temporal hierarchy of gene expression mediated by transcription factor binding affinity and activation dynamics. MBio 6(3):e00686-15
García Véscovi E, Soncini FC, Groisman EA (1996) Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84(1):165–174
Gardner SG, Johns KD, Tanner R, McCleary WR (2014) The PhoU protein from Escherichia coli interacts with PhoR, PstB, and metals to form a phosphate-signaling complex at the membrane. J Bacteriol 196(9):1741–1752
Gardner SG, McCleary WR (2019) Control of the phoBR regulon in Escherichia coli. EcoSal Plus 8(2)
Gillooly JF, Allen AP, Brown JH, Elser JJ, Martinez del Rio C, Savage VM et al (2005) The metabolic basis of whole-organism RNA and phosphorus content. Proc Natl Acad Sci U S A 102(33):11923–11927
Glover RT, Kriakov J, Garforth SJ, Baughn AD, Jacobs WR Jr (2007) The two-component regulatory system senX3-regX3 regulates phosphate-dependent gene expression in Mycobacterium smegmatis. J Bacteriol 189(15):5495–5503
Groisman EA (2016) Feedback control of two-component regulatory systems. Annu Rev Microbiol 70:103–124
Groisman EA, Hollands K, Kriner MA, Lee EJ, Park SY, Pontes MH (2013) Bacterial Mg2+ homeostasis, transport, and virulence. Annu Rev Genet 47:625–646
Groisman EA, Kato A (2008) The PhoQ/PhoP regulatory network of Salmonella enterica. Adv Exp Med Biol 631:7–21
Guo L, Lim KB, Poduje CM, Daniel M, Gunn JS, Hackett M et al (1998) Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95(2):189–198
Harris RM, Webb DC, Howitt SM, Cox GB (2001) Characterization of PitA and PitB from Escherichia coli. J Bacteriol 183(17):5008–5014
Hengge R (2021) High-specificity local and global c-di-GMP signaling. Trends Microbiol:S0966-842X(21)00037-8
Hernando N, Gagnon K, Lederer E (2021) Phosphate transport in epithelial and nonepithelial tissue. Physiol Rev 101(1):1–35
Hirota R, Motomura K, Nakai S, Handa T, Ikeda T, Kuroda A (2013) Stable polyphosphate accumulation by a pseudo-revertant of an Escherichia coli phoU mutant. Biotechnol Lett 35(5):695–701
Hoffer SM, Tommassen J (2001) The phosphate-binding protein of Escherichia coli is not essential for Pi-regulated expression of the Pho regulon. J Bacteriol 183(19):5768–5771
Hollands K, Proshkin S, Sklyarova S, Epshtein V, Mironov A, Nudler E et al (2012) Riboswitch control of Rho-dependent transcription termination. Proc Natl Acad Sci U S A 109(14):5376–5381
Hong X, Chen HD, Groisman EA (2018) Gene expression kinetics governs stimulus-specific decoration of the Salmonella outer membrane. Sci Signal 11(529):eaar7921
Hulett FM, Lee J, Shi L, Sun G, Chesnut R, Sharkova E et al (1994) Sequential action of two-component genetic switches regulates the PHO regulon in Bacillus subtilis. J Bacteriol 176(5):1348–1358
Irving SE, Choudhury NR, Corrigan RM (2021) The stringent response and physiological roles of (pp)pGpp in bacteria. Nat Rev Microbiol 19(4):256–271
Jackson RJ, Binet MR, Lee LJ, Ma R, Graham AI, McLeod CW et al (2008) Expression of the PitA phosphate/metal transporter of Escherichia coli is responsive to zinc and inorganic phosphate levels. FEMS Microbiol Lett 289(2):219–224
Jensen LT, Ajua-Alemanji M, Culotta VC (2003) The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J Biol Chem 278(43):42036–42040
Jiang J, Yu K, Qi L, Liu Y, Cheng S, Wu M et al (2018) A proteomic view of Salmonella Typhimurium in response to phosphate limitation. Proteomes 6(2):19
Kelliher JL, Radin JN, Grim KP, Párraga Solórzano PK, Degnan PH, Kehl-Fie TE (2018) Acquisition of the phosphate transporter NptA enhances Staphylococcus aureus pathogenesis by improving phosphate uptake in divergent environments. Infect Immun 86(1):e00631–e00617
Kimes BW, Morris DR (1973) Cations and ribosome structure. II. Effects on the 50S subunit of substituting polyamines for magnesium ion. Biochemistry 12(3):442–449
Klein DJ, Moore PB, Steitz TA (2004) The contribution of metal ions to the structural stability of the large ribosomal subunit. RNA 10(9):1366–1379
Klein G, Müller-Loennies S, Lindner B, Kobylak N, Brade H, Raina S (2013) Molecular and structural basis of inner core lipopolysaccharide alterations in Escherichia coli: incorporation of glucuronic acid and phosphoethanolamine in the heptose region. J Biol Chem 288(12):8111–8127
Lebens M, Lundquist P, Söderlund L, Todorovic M, Carlin NI (2002) The nptA gene of Vibrio cholerae encodes a functional sodium-dependent phosphate cotransporter homologous to the type II cotransporters of eukaryotes. J Bacteriol 184(16):4466–4474
Lee EJ, Groisman EA (2012a) Tandem attenuators control expression of the Salmonella mgtCBR virulence operon. Mol Microbiol 86(1):212–224
Lee EJ, Groisman EA (2012b) Control of a Salmonella virulence locus by an ATP-sensing leader messenger RNA. Nature 486(7402):271–275
Lee EJ, Pontes MH, Groisman EA (2013) A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium’ s own F1Fo ATP synthase. Cell 154(1):146–156
Lejona S, Aguirre A, Cabeza ML, García Véscovi E, Soncini FC (2003) Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica. J Bacteriol 185(21):6287–6294
Lejona S, Castelli ME, Cabeza ML, Kenney LJ, García Véscovi E, Soncini FC (2004) PhoP can activate its target genes in a PhoQ-independent manner. J Bacteriol 186(8):2476–2480
Lippa AM, Goulian M (2009) Feedback inhibition in the PhoQ/PhoP signaling system by a membrane peptide. PLoS Genet 5(12):e1000788
Liu W, Eder S, Hulett FM (1998) Analysis of Bacillus subtilis tagAB and tagDEF expression during phosphate starvation identifies a repressor role for PhoP~P. J Bacteriol 180(3):753–758
Lubin EA, Henry JT, Fiebig A, Crosson S, Laub MT (2016) Identification of the PhoB regulon and role of PhoU in the phosphate starvation response of Caulobacter crescentus. J Bacteriol 198(1):187–200
Madigan MT, Martinko JM, Bender KS, Buckley DH (2015) Microbial metabolism. In: Madigan MT, Martinko JM, Bender KS et al (eds) Brock biology of microorganisms, 14th edn. Pearson, London, pp 73–105
Maguire ME, Cowan JA (2002) Magnesium chemistry and biochemistry. Biometals 15(3):203–210
Makino K, Amemura M, Kawamoto T, Kimura S, Shinagawa H, Nakata A et al (1996) DNA binding of PhoB and its interaction with RNA polymerase. J Mol Biol 259(1):15–26
Makino K, Shinagawa H, Amemura M, Kawamoto T, Yamada M, Nakata A (1989) Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J Mol Biol 210(3):551–559
Makino K, Shinagawa H, Amemura M, Kimura S, Nakata A, Ishihama A (1988) Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol 203(1):85–95
Masotti F, Garavaglia BS, Piazza A, Burdisso P, Altabe S, Gottig N et al (2021) Bacterial isolates from Argentine Pampas and their ability to degrade glyphosate. Sci Total Environ 774:145761
McCarthy S, Ai C, Wheaton G, Tevatia R, Eckrich V, Kelly R et al (2014) Role of an archaeal PitA transporter in the copper and arsenic resistance of Metallosphaera sedula, an extreme thermoacidophile. J Bacteriol 196(20):3562–3570
Medveczky N, Rosenberg H (1971) Phosphate transport in Escherichia coli. Biochim Biophys Acta 241(2):494–506
Morohoshi T, Maruo T, Shirai Y, Kato J, Ikeda T, Takiguchi N et al (2002) Accumulation of inorganic polyphosphate in phoU mutants of Escherichia coli and Synechocystis sp. strain PCC6803. Appl Environ Microbiol 68(8):4107–4110
Murray EL, Conway T (2005) Multiple regulators control expression of the Entner-Doudoroff aldolase (Eda) of Escherichia coli. J Bacteriol 187(3):991–1000
Novak R, Cauwels A, Charpentier E, Tuomanen E (1999) Identification of a Streptococcus pneumoniae gene locus encoding proteins of an ABC phosphate transporter and a two-component regulatory system. J Bacteriol 181(4):1126–1133
Ofiteru AM, Ruta LL, Rotaru C, Dumitru I, Ene CD, Neagoe A et al (2012) Overexpression of the PHO84 gene causes heavy metal accumulation and induces Ire1p-dependent unfolded protein response in Saccharomyces cerevisiae cells. Appl Microbiol Biotechnol 94(2):425–435
Park SY, Cromie MJ, Lee EJ, Groisman EA (2010) A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell 142(5):737–748
Park SY, Groisman EA (2014) Signal-specific temporal response by the Salmonella PhoP/PhoQ regulatory system. Mol Microbiol 91(1):135–144
Perez JC, Shin D, Zwir I, Latifi T, Hadley TJ, Groisman EA (2009) Evolution of a bacterial regulon controlling virulence and Mg2+ homeostasis. PLoS Genet 5(3):e1000428
Persson BL, Petersson J, Fristedt U, Weinander R, Berhe A, Pattison J (1999) Phosphate permeases of Saccharomyces cerevisiae: structure, function and regulation. Biochim Biophys Acta 1422(3):255–272
Petrov AS, Bowman JC, Harvey SC, Williams LD (2011) Bidentate RNA-magnesium clamps: on the origin of the special role of magnesium in RNA folding. RNA 17(2):291–297
Pontes MH, Groisman EA (2018) Protein synthesis controls phosphate homeostasis. Genes Dev 32(1):79–92
Pontes MH, Lee E-J, Choi J, Groisman EA (2015a) Salmonella promotes virulence by repressing cellulose production. Proc Natl Acad Sci U S A 112(16):5183–5188
Pontes MH, Sevostyanova A, Groisman EA (2015b) When too much ATP is bad for protein synthesis. J Mol Biol 427(16):2586–2594
Pontes MH, Yeom J, Groisman EA (2016) Reducing ribosome biosynthesis promotes translation during low Mg2+stress. Mol Cell 64(3):480–492
Rao NN, Roberts MF, Torriani A, Yashphe J (1993) Effect of glpT and glpD mutations on expression of the phoA gene in Escherichia coli. J Bacteriol 175(1):74–79
Rao NN, Torriani A (1990) Molecular aspects of phosphate transport in Escherichia coli. Mol Microbiol 4(7):1083–1090
Rice CD, Pollard JE, Lewis ZT, McCleary WR (2009) Employment of a promoter-swapping technique shows that PhoU modulates the activity of the PstSCAB2 ABC transporter in Escherichia coli. Appl Environ Microbiol 75(3):573–582
Rosenberg H, Gerdes RG, Chegwidden K (1977) Two systems for the uptake of phosphate in Escherichia coli. J Bacteriol 131(2):505–511
Rosenfeld L, Culotta VC (2012) Phosphate disruption and metal toxicity in Saccharomyces cerevisiae: effects of RAD23 and the histone chaperone HPC2. Biochem Biophys Res Commun 418(2):414–419
Salzberg LI, Botella E, Hokamp K, Antelmann H, Maaß S, Becher D et al (2015) Genome-wide analysis of phosphorylated PhoP binding to chromosomal DNA reveals several novel features of the PhoPR-mediated phosphate limitation response in Bacillus subtilis. J Bacteriol 197(8):1492–1506
Scholten M, Tommassen J (1993) Topology of the PhoR protein of Escherichia coli and functional analysis of internal deletion mutants. Mol Microbiol 8(2):269–275
Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM et al (2005) Structures of the bacterial ribosome at 3.5 A resolution. Science 310(5749):827–834
Sevostyanova A, Groisman EA (2015) An RNA motif advances transcription by preventing Rho-dependent termination. Proc Natl Acad Sci U S A 112(50):E6835–E6843
Shi L, Liu W, Hulett FM (1999) Decay of activated Bacillus subtilis Pho response regulator, PhoP~P, involves the PhoR~P intermediate. Biochemistry 38(31):10119–10125
Shi Y, Cromie MJ, Hsu FF, Turk J, Groisman EA (2004) PhoP-regulated Salmonella resistance to the antimicrobial peptides magainin 2 and polymyxin B. Mol Microbiol 53(1):229–241
Shin D, Groisman EA (2005) Signal-dependent binding of the response regulators PhoP and PmrA to their target promoters in vivo. J Biol Chem 280(6):4089–4094
Snavely MD, Florer JB, Miller CG, Maguire ME (1989) Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J Bacteriol 171(9):4761–4766
Snavely MD, Miller CG, Maguire ME (1991) The mgtB Mg2+ transport locus of Salmonella typhimurium encodes a P-type ATPase. J Biol Chem 266(2):815–823
Soncini FC, García Véscovi E, Solomon F, Groisman EA (1996) Molecular basis of the magnesium deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J Bacteriol 178(17):5092–5099
Spinelli SV, Pontel LB, García Véscovi E, Soncini FC (2008) Regulation of magnesium homeostasis in Salmonella: Mg2+ targets the mgtA transcript for degradation by RNase E. FEMS Microbiol Lett 280(2):226–234
Steed PM, Wanner BL (1993) Use of the rep technique for allele replacement to construct mutants with deletions of the pstSCAB-phoU operon: evidence of a new role for the PhoU protein in the phosphate regulon. J Bacteriol 175(21):6797–6809
Storer AC, Cornish-Bowden A (1976) Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J 159(1):1–5
Stouthamer AH (1973) A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek 39(3):545–565
Surin BP, Rosenberg H, Cox GB (1985) Phosphate-specific transport system of Escherichia coli: Nucleotide sequence and gene-polypeptide relationships. J Bacteriol 161(1):189–198
Tao T, Snavely MD, Farr SG, Maguire ME (1995) Magnesium transport in Salmonella typhimurium: mgtA encodes a P-type ATPase and is regulated by Mg2+ in a manner similar to that of the mgtB P-type ATPase. J Bacteriol 177(1):2654–2662
Trent MS, Pabich W, Raetz CRH, Miller SI (2001) A PhoP/PhoQ-induced Lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J Biol Chem 276(12):9083–9092
van Veen HW (1997) Phosphate transport in prokaryotes: molecules, mediators and mechanisms. Antonie van Leeuwenhoek Int J Gen Mol Microbiol 72(4):299–315
van Veen HW, Abee T, Kortstee GJ, Konings WN, Zehnder AJ (1994) Translocation of metal phosphate via the phosphate inorganic transport system of Escherichia coli. Biochemistry 33(7):1766–1770
Vila-Costa M, Sebastián M, Pizarro M, Cerro-Gálvez E, Lundin D, Gasol JM et al (2019) Microbial consumption of organophosphate esters in seawater under phosphorus limited conditions. Sci Rep 9(1):233
Wacker WE (1969) The biochemistry of magnesium. Ann N Y Acad Sci 162(2):717–726
Wang C, Mao Y, Yu J, Zhu L, Li M, Wang D et al (2013) PhoY2 of mycobacteria is required for metabolic homeostasis and stress response. J Bacteriol 195(2):243–252
Wang X, Han H, Lv Z, Lin Z, Shang Y, Xu T et al (2017) PhoU2 but not PhoU1 as an important regulator of biofilm formation and tolerance to multiple stresses by participating in various fundamental metabolic processes in Staphylococcus epidermidis. J Bacteriol 199(24):e00219–e00217
Wanner BL (1993) Gene regulation by phosphate in enteric bacteria. J Cell Biochem 51(1):47–54
Wanner BL (1996) Phosphorus assimilation and control of the phosphate regulon. In: Neidhardt FC, Curtiss R, Ingraham JL et al (eds) Escherichia coli and Salmonella: cellular and molecular biology, 2nd edn. American Society for Microbiology, Washington, DC, pp 1357–1381
Wanner BL, Hsieh Y-J (2010) Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 13(2):198–203
Webb DC, Rosenberg H, Cox GB (1992) Mutational analysis of the Escherichia coli phosphate-specific transport system, a member of the traffic ATPase (or ABC) family of membrane transporters. A role for proline residues in transmembrane helices. J Biol Chem 267(34):24661–24668
Weiss RL, Kimes BW, Morris DR (1973) Cations and ribosome structure. 3. Effects on the 30S and 50S subunits of replacing bound Mg2+ by inorganic cations. Biochemistry 12(3):450–456
Weiss RL, Morris DR (1973) Cations and ribosome structure. I. Effects on the 30S subunit of substituting polyamines for magnesium ion. Biochemistry 12(3):435–441
Werner A, Kinne RKH (2001) Evolution of the Na-Pi cotransport systems. Am J Phys Regul Integr Comp Phys 280(2):R301–R312
Wilkins AS (1972) Physiological factors in the regulation of alkaline phosphatase synthesis in Escherichia coli. J Bacteriol 110(2):616–623
Willsky GR, Malamy MH (1980a) Characterization of two genetically separable inorganic phosphate transport systems in Escherichia coli. J Bacteriol 144(1):356–365
Willsky GR, Malamy MH (1980b) Effect of arsenate on inorganic phosphate transport in Escherichia coli. J Bacteriol 144(1):366–374
Wösten MM, Parker CT, van Mourik A, Guilhabert MR, van Dijk L, van Putten JP (2006) The Campylobacter jejuni PhosS/PhosR operon represents a non-classical phosphate-sensitive two-component system. Mol Microbiol 62(1):278–291
Wuttge S, Bommer M, Jäger F, Martins BM, Jacob S, Licht A et al (2012) Determinants of substrate specificity and biochemical properties of the sn-glycerol-3-phosphate ATP binding cassette transporter (UgpB-AEC2) of Escherichia coli. Mol Microbiol 86(4):908–920
Yamamoto K, Ogasawara H, Fujita N, Utsumi R, Ishihama A (2002) Novel mode of transcription regulation of divergently overlapping promoters by PhoP, the regulator of two-component system sensing external magnesium availability. Mol Microbiol 45(2):423–438
Yang C, Huang TW, Wen SY, Chang CY, Tsai SF, Wu WF et al (2012) Genome-wide PhoB binding and gene expression profiles reveal the hierarchical gene regulatory network of phosphate starvation in Escherichia coli. PLoS One 7(10):e47314
Yeom J, Shao Y, Groisman EA (2020) Small proteins regulate Salmonella survival inside macrophages by controlling degradation of a magnesium transporter. Proc Natl Acad Sci U S A 117(33):20235–20243
Yin X, Wu Orr M, Wang H, Hobbs EC, Shabalina SA, Storz G (2019) The small protein MgtS and small RNA MgrR modulate the PitA phosphate symporter to boost intracellular magnesium levels. Mol Microbiol 111(1):131-144.
Yoshida Y, Sugiyama S, Oyamada T, Yokoyama K, Makino K (2012) Novel members of the phosphate regulon in Escherichia coli O157: H7 identified using a whole-genome shotgun approach. Gene 502(1):27–35
Zarrella TM, Bai G (2020) The many roles of the bacterial second messenger cyclic di-AMP in adapting to stress cues. J Bacteriol 203(1):e00348–e00320
Zheng JJ, Sinha D, Wayne KJ, Winkler ME (2016) Physiological roles of the dual phosphate transporter systems in low and high phosphate conditions and in capsule maintenance of Streptococcus pneumoniae D39. Front Cell Infect Microbiol 6:63
Zwir I, Latifi T, Perez JC, Huang H, Groisman EA (2012) The promoter architectural landscape of the Salmonella PhoP regulon. Mol Microbiol 84(3):463–485
Zwir I, Shin D, Kato A, Nishino K, Latifi T, Solomon F et al (2005) Dissecting the PhoP regulatory network of Escherichia coli and Salmonella enterica. Proc Natl Acad Sci U S A 102(8):2862–2867
Zwir I, Yeo WS, Shin D, Latifi T, Huang H, Groisman EA (2014) Bacterial nucleoid-associated protein uncouples transcription levels from transcription timing. MBio 5(5):e01485-14
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bruna, R.E., Kendra, C.G., Pontes, M.H. (2022). Coordination of Phosphate and Magnesium Metabolism in Bacteria. In: Razzaque, M.S. (eds) Phosphate Metabolism . Advances in Experimental Medicine and Biology, vol 1362. Springer, Cham. https://doi.org/10.1007/978-3-030-91623-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-91623-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91621-3
Online ISBN: 978-3-030-91623-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)