Abstract
The rapidly changing global climate due to increased emission of anthropogenic greenhouse gases (GHGs) is leading to an increased occurrence of extreme weather events such as droughts, floods, and heatwaves. The three major GHGs are carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O). The major natural sources of CO2 include ocean–atmosphere exchange, respiration of animals, soils (microbial respiration) and plants, and volcanic eruption; while the anthropogenic sources include burning of fossil fuel (coal, natural gas, and oil), deforestation, and the cultivation of land that increases the decomposition of soil organic matter and crop and animal residues. Natural sources of CH4 emission include wetlands, termite activities, and oceans. Paddy fields used for rice production, livestock production systems (enteric emission from ruminants), landfills, and the production and use of fossil fuels are the main anthropogenic sources of CH4. Nitrous oxide, in addition to being a major GHG, is also an ozone-depleting gas. N2O is emitted by natural processes from oceans and terrestrial ecosystems. Anthropogenic N2O emissions occur mostly through agricultural and other land-use activities and are associated with the intensification of agricultural and other human activities such as increased use of synthetic fertiliser (119.4 million tonnes of N worldwide in 2019), inefficient use of irrigation water, deposition of animal excreta (urine and dung) from grazing animals, excessive and inefficient application of farm effluents and animal manure to croplands and pastures, and management practices that enhance soil organic N mineralisation and C decomposition. Agriculture could act as a source and a sink of GHGs. Besides direct sources, GHGs also come from various indirect sources, including upstream and downstream emissions in agricultural systems and ammonia (NH3) deposition from fertiliser and animal manure.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
The global climate is changing rapidly. This leads to the increasing occurrence of extreme weather events such as droughts and floods. The major cause of these events is the rising temperature in the Earth’s atmosphere, which is driven by increasing emissions of climate-relevant greenhouse gases (GHGs) that trap heat in the atmosphere. Major GHGs include carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O) (Fig. 1.1).
Carbon dioxide is the major GHG responsible for the increasing greenhouse effect of the atmosphere. Key natural sources of CO2 include ocean–atmosphere exchange, respiration of animals, soils (microbial respiration) and plants, and volcanic eruption. Major anthropogenic sources of CO2 include burning of fossil fuel (coal, natural gas, and oil), deforestation, and the cultivation of land that increases the decomposition of soil organic matter and crop and animal residues (Xu and Shang 2016).
Aside from CO2, CH4 is a major GHG, which is emitted by natural and anthropogenic processes. Natural sources of CH4 emission include wetlands, termite activities, and occan. Paddy fields used for rice production, livestock production systems (enteric emission from ruminants), landfills, and production and use of fossil fuels are the main anthropogenic sources of CH4. Furthermore, CH4 can be produced by anaerobic mineralization by methanogenic archaea in both natural and man-made systems. Also, plants have been shown to emit CH4.
The third major GHG is N2O (Zaman et al. 2012). Besides being a major GHG, N2O is a major ozone-depleting gas (Ravishankara et al. 2009). Oceans and soils under natural vegetation are non-anthropogenic sources of N2O. However, at a global scale, the emission of N2O is mostly caused by, or related to, anthropogenic agricultural and other land-use activities. The atmospheric concentration of N2O has increased by more than 20% from ~271 ppb to 331 ppb since the industrial era (ca. 1750) to 2018 (WMO 2019). Over the last decade, the rate of N2O increase was equal to 0.95 ppb yr−1 (IPCC 2013b; WMO 2019) with an increasing trend (Makowski 2019; Thompson et al. 2019). In 2006, the total anthropogenic source of N2O was 6.9 Tg N2O-N. Out of these direct emissions, agricultural sources dominated (4.1 Tg N2O-N), while indirect emissions accounted for 0.6 (with a range of 0.1–2.9) Tg N2O-N (IPCC 2013a). Such a large N2O emission is attributed to various factors including the intensification of agricultural and other human activities, increased use of synthetic fertiliser (119.4 million tonnes of N worldwide in 2019), inefficient use of irrigation water, deposition of animal excreta (urine and dung) from grazing animals, excessive and inefficient application of farm effluents and animal manure to croplands and pastures, and management practices that enhance soil organic N mineralisation and C decomposition. These activities affect the N cycle. The N cycle is rather complex, and it has even been disrupted due to increased N inputs and intensification of agriculture. Sources of increased N inputs, in particular reactive N, into the N cycle stem, for instance, from the Haber–Bosch process (Erisman et al. 2011), thereby transforming the N cycle into the so-called N cascade, which is characterised by the release of reactive N forms into the environment with various consequences (Sutton et al. 2011). There are still many uncertainties concerning the N cycle. For example, the role of individual factors controlling the occurrence and rate of the key N transformation processes, such as denitrification and nitrification, is uncertain (Butterbach-Bahl et al. 2013; Müller and Clough 2014; Smith 2017). Nitrification results in ammonium (NH4+) being converted to nitrate (NO3−) under aerobic conditions, while denitrification is the reduction of NO3− to N2 under anaerobic conditions. Nitrifier denitrification occupies the niche between nitrification and denitrification and occurs as oxygen concentrations approach an anaerobic status. Under these conditions, nitrifiers actually convert nitrite into N2O and N2 instead of nitrate (Wrage-Mönnig et al. 2018).
The three GHGs (CO2, CH4, and N2O), derived from various sectors, play a major role in regulating Earth’s temperature (Fig. 1.1). Without GHGs in the atmosphere, the average global soil surface temperature would be ~19 ℃, compared to the present values of 14 ℃ (Hossain 2019).
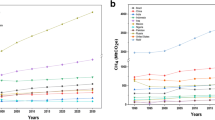
Recent data from the UN Intergovernmental Panel on Climate Change (IPCC) clearly show that anthropogenic emissions of GHGs are at the highest in history (IPCC 2014a). Since 1990, Earth’s average surface air temperature has increased by about 0.8 ℃, with much of the emission increases taking place since the mid-1970s (Fig. 1.2).
The global warming potential (GWP) of a GHG relates to the amount of heat trapped by a certain mass of a gas to the amount of heat trapped by a similar mass of CO2 calculated over a 100-year time horizon (IPCC 2016). For example, the GWP of N2O is 265–298, which means if the same masses of N2O and CO2 were emitted into the atmosphere, N2O would trap 265–298 times more heat than CO2 over a 100-year time period (Table 1.1) (IPCC 2016).
The Kyoto Protocol was negotiated by parties to the United Nations Framework Convention on Climate Change (UNFCCC) in an effort to stabilise the continued increase in atmospheric GHG concentrations. The Kyoto protocol outlines GHG reduction targets for participating countries. The signatories to the protocol must develop and report on their annual national inventory of anthropogenic GHG emissions. Guidelines on how to construct inventories were prescribed by the IPCC (2014b). Country-specific emission data can be considered but that requires accurate inventory data based on precise GHG measurements.
1.2 Impact of Ammonia on GHG Emissions
Besides direct sources, GHGs also come from different indirect sources such as upstream and downstream in agricultural systems (Plate 1.1).
Schematic representation of direct (from the cropping system) and indirect (upstream and downstream) GHG emissions from crop production. As an example, ammonia emitted from a cropping system will be deposited and potentially oxidised to nitrate, which can further be denitrified, thus enhancing the risk for N2O emission (Aguilera Fernández 2016)
Ammonia (NH3) itself has no direct greenhouse effect. It is a gas with a relatively short residence time in the atmosphere (2–10 days) compared to some GHGs, such as CO2 (3–4 years), CH4 (12 years), and N2O (114 years). However, after NH3 is emitted to the atmosphere and reacts with acids, it forms salts. These salts then return to The Earth’s surface and act as a N source source for N2O emissions, similar to a fertiliser-N application. When the soil is submitted to conditions near the optimum for urease activity (e.g. pH close to neutrality, soil moisture near field capacity, temperature >30 ℃), the N losses through NH3 volatilisation from urea-based fertilisers applied on soil surface can be as high as 50% (Martins et al. 2017; Rochette et al. 2013). Therefore, the measurement of NH3 emission is important to estimate indirect N2O emissions derived from soil amendments, such as urea-based fertilisers, green manures, animal excreta, or ammonium-based fertilisers in alkaline soils. A default emission factor defined by IPCC, known as EF4, can be applied for the estimation of indirect N2O emissions derived from volatilisation of NH3 and other nitrogen oxides (NOx) (IPCC 2006). The mean value of EF4, considering the N volatilisation and consequent re-deposition, is 0.01 kg N2O-N per kg N volatilised as NH3 with an uncertainty ranging from 0.002 to 0.05 (IPCC 2006). Therefore, management options that reduce NH3 volatilisation from soils are considered mitigation practices because they reduce indirect N2O emissions (IPCC 2014a; Lam et al. 2017).
1.3 Aim of the Book
In 1992, the International Atomic Energy Agency (IAEA) published a “Manual on measurement of CH4 and N2O emissions from agriculture” (IAEA 1992). Since the publication of the manual, progress has been made in analytical techniques. The progress includes advances in automation technologies as well as the theoretical understanding of how soil microbial processes affect CH4 and N2O emissions and the factors influencing those microbial processes.
Hence, the aim of this book is to provide an updated account of the state-of-the-art techniques to measure direct GHG emissions (Plate 1.1), as a necessary step to propose and assess any mitigation strategy. The focus is on CH4 and N2O emissions. Additionally, information on techniques to measure indirect GHG sources is provided. Indirect GHG sources in this book include volatilised ammonia (NH3). NH3 is a reactive N gas highly affecting the environment through eutrophication and acidification of natural ecosystems as well as human health due to the promotion of particulate matter formation (Sanz Cobena et al. 2014). Moreover, in line with the 1992 IAEA Tecdoc document, the hands-on approach is also followed here so that researchers, who want to use the techniques described in this book, can easily apply them to their own work.
References
Aguilera Fernández EM (2016) The influence of management practices on the greenhouse gas balance of Mediterranean cropping systems. Identifying the climate change mitigation potential through quantitative review and life cycle assessment. Departmento de Geografía, Historia y filosofía laboratorio de historia de los agroecosistemas. Universidad Pablo de Olavide, Sevilla, pp 428
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils, how well do we understand the processes and their controls. Philos T R Soc London B 368:16–21
Erisman JW, Galloway J, Seitzinger S, Bleeker A, Butterbach-Bahl K (2011) Reactive nitrogen in the environment and its effect on climate change. Curr Opin Environ Sustain 3:281–295
Hossain F (2019) Sustainable design and build building, energy, roads, bridges, water and sewer systems. Butterworth-Heinemann, Elsevier, p 462
IAEA (1992) Manual on measurement of methane and nitrous oxide emissions from agriculture. International Atomic Energy Agency IAEA, Vienna, p 90
IPCC (2006) 2006 IPCC guidelines for national greenhouse gas inventories Chapter 11 N2O emissions from managed soils, and CO2 emissions from lime and urea application, 2006 IPCC guidelines for national greenhouse gas inventories
IPCC (2013a) Climate change 2013: The physical science basis. In: Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. United Kingdom and New York, pp 1535
IPCC (2013b) Working group I contribution to the IPCC fifth assessment report climate change 2013: The physical science basis summary for policymakers. Intergovernmental Panel for Climate Change, pp 36
IPCC (2014a) Climate change 2014 synthesis report: Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. In: Team TCW, Pachauri RK, Meyer LA (eds) A report of the intergovernmental panel on climate change. Geneva, Switzerland, pp 151
IPCC (2014b) Supplement to the 2006 IPCC guidelines for national greenhouse gas inventories: Wetlands, In: Hirashi T, Krug T, Tanabe K, Srivastava N, Baasansuren J, Fukuda M, Troxler TG (eds) IPCC task force on national greenhouse gas inventories. Switzerland, pp 354
IPCC (2016) Global warming potential values on climate change 2013: The physical science basis. In: Contribution of working group I fifth assessment report of the intergovernmental panel on climate change, pp 4
Lam SK, Suter H, Mosier AR, Chen D (2017) Using nitrification inhibitors to mitigate agricultural N2O emission: a double-edged sword? Glob Chang Biol 23:485–489
Makowski D (2019) N2O increasing faster than expected. Nat Clim Chang 9:909–910
Martins MR, Sant’Anna SAC, Zaman M, Santos RC, Monteiro RC, Alves BJR, Jantalia CP, Boddey RM, Urquiaga S (2017) Strategies for the use of urease and nitrification inhibitors with urea: Impact on N2O and NH3 emissions, fertilizer-15N recovery and maize yield in a tropical soil. Agric Ecosy Environ 247:54–62
Müller C, Clough TJ (2014) Advances in understanding nitrogen flows and transformations: gaps and research pathways. J Agric Sci Cambridge 152:S34–S44
Ravishankara AR, Daniel JS, Portmann RW (2009) Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125
Rochette P, Angers DA, Chantigny MH, Gasser MO, MacDonald JD, Pelster DE, Bertrand N (2013) NH3 volatilization, soil NH4+ concentration and soil pH following subsurface banding of urea at increasing rates. Can J Soil Sci 93:261–268
Sanz Cobena A, Lassaletta L, Estellés F, Del Prado A, Guardia G, Abalos D, Aguilera Fernández EM, Pardo G, Vallejo A, Sutton MA, Garnier J, Billen G (2014) Yield-scaled mitigation of ammonia emission from N fertilization: the Spanish case. Environ Res Lett 9(125012): 125005
Smith KA (2017) Changing views of nitrous oxide emissions from agricultural soil: key controlling processes and assessment at different spatial scales. Eur J Soil Sci 68:137–155
Sutton MA, Oenema O, Erisman JW, Leip A, van Grinsven H, Winiwarter W (2011) Too much of a good thing. Nature 472:159–161
Thompson RL, Lassaletta L, Patra PK, Wilson C, Wells KC, Gressent A, Koffi EN, Chipperfield MP, Winiwarter W, Davidson EA, Tian H, Canadell JG (2019) Acceleration of global N2O emissions seen from two decades of atmospheric inversion. Nat Clim Chang 9:993–998
WMO (2019) The state of greenhouse gases in the atmosphere based on global observations through 2018. WMO Greenhouse Gas Bulletin 15:1–8
Wrage-Mönnig N, Horn MA, Well R, Müller C, Velthof G, Oenema O (2018) The role of nitrifier denitrification in the production of nitrous oxide revisited. Soil Biol Biochem 123:A3–A16
Xu M, Shang H (2016) Contribution of soil respiration to the global carbon equation. J Plant Physio 203:16–28
Zaman M, Nguyen ML, Šimek M, Nawaz S, Khan MJ, Babar MN, and Zaman S (2012) Emissions of nitrous oxide (N2O) and di-nitrogen (N2) from agricultural landscape, sources, sinks, and factors affecting N2O and N2 ratios. In: Guoxiang Liu (ed) Greenhouse gases-emission, measurement and management. Intech, Croatia, pp 1–32
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
The opinions expressed in this chapter are those of the author(s) and do not necessarily reflect the views of the International Atomic Energy Agency, its Board of Directors, or the countries they represent
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 3.0 IGO license (http://creativecommons.org/licenses/by/3.0/igo/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the International Atomic Energy Agency, provide a link to the Creative Commons license and indicate if changes were made.
Any dispute related to the use of the works of the International Atomic Energy Agency that cannot be settled amicably shall be submitted to arbitration pursuant to the UNCITRAL rules. The use of the International Atomic Energy Agency's name for any purpose other than for attribution, and the use of the International Atomic Energy Agency's logo, shall be subject to a separate written license agreement between the International Atomic Energy Agency and the user and is not authorized as part of this CC-IGO license. Note that the link provided above includes additional terms and conditions of the license.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Zaman, M. et al. (2021). Greenhouse Gases from Agriculture. In: Zaman, M., Heng, L., Müller, C. (eds) Measuring Emission of Agricultural Greenhouse Gases and Developing Mitigation Options using Nuclear and Related Techniques. Springer, Cham. https://doi.org/10.1007/978-3-030-55396-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-55396-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-55395-1
Online ISBN: 978-3-030-55396-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)