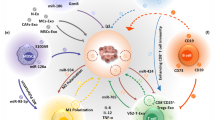
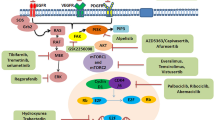
Abstract
Neuroblastoma is a solid tumor (a lump or mass), often found in the small glands on top of the kidneys, and most commonly affects infants and young children. Among neuroblastomas, high-risk neuroblastomas are very aggressive and resistant to most kinds of intensive treatment. Immunotherapy, which uses the immune system to fight against cancer, has shown great promise in treating many types of cancer. However, high-risk neuroblastoma is often resistant to this approach as well. Recent studies revealed that small vesicles known as exosomes, which are envelopes, could deliver a cargo of small RNA molecules and provide communication between neuroblastoma cells and the surrounding cells and trigger metastasis and resistance to immunotherapy. In this chapter, we describe the role of exosomes and small RNA molecules in the metastasis and regression of neuroblastoma and the potential therapeutic approaches to combat this menace.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- 3′-UTR :
-
Three prime untranslated region
- ADCC :
-
Antibody-dependent cell cytotoxicity
- AURKA:
-
Aurora kinase A
- EFS:
-
Event-free survival
- ESCRT:
-
Endosomal-sorting complex required for transport
- GD2:
-
Disialoganglioside
- IL-15:
-
Interleukin-15
- IL-2:
-
Interleukin-2
- ILV:
-
Intraluminal vesicle
- MAb :
-
Monoclonal antibody
- miRNA:
-
MicroRNA
- mRNA:
-
Messenger RNA
- MSCs:
-
Mesenchymal stem/stromal cells
- MVBs :
-
Multivesicular bodies
- MYCN :
-
v-myc myelocytomatosis viral-related oncogene, neuroblastoma-derived
- NEDD4:
-
Neuronal precursor cell-expressed developmentally downregulated 4
- NF-κB:
-
Nuclear factor-kappa B
- NK :
-
Natural killer
- PCR:
-
Polymerase chain reaction
- PNTs :
-
Peripheral neuroblastic tumors
- RNA :
-
Ribonucleic acid
- TERF1:
-
Telomeric repeat-binding factor 1
- TGFβ 1:
-
Transforming growth factor beta 1
- TGFβR1:
-
Transforming growth factor beta receptor 1
- TGFβR2:
-
Transforming growth factor beta receptor 2
- TLR8 :
-
Toll-like receptor 8
References
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN et al (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10(3):301–312. https://doi.org/10.1016/j.scr.2013.01.002
Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA et al (2009) Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med 180(11):1122–1130. https://doi.org/10.1164/rccm.200902-0242OC
Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton A et al (2010) Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med 363(14):1313–1323. https://doi.org/10.1056/NEJMoa1001527
Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A et al (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307(1):C25–C38. https://doi.org/10.1152/ajpcell.00084.2014
Bobrie A, Colombo M, Raposo G, Thery C (2011) Exosome secretion: molecular mechanisms and roles in immune responses. Traffic 12(12):1659–1668. https://doi.org/10.1111/j.1600-0854.2011.01225.x
Brummer A, Hausser J (2014) MicroRNA binding sites in the coding region of mRNAs: extending the repertoire of post-transcriptional gene regulation. BioEssays 36(6):617–626. https://doi.org/10.1002/bies.201300104
Challagundla KB, Wise PM, Neviani P, Chava H, Murtadha M, Xu T et al (2015) Exosome-mediated transfer of microRNAs within the tumor microenvironment and neuroblastoma resistance to chemotherapy. J Natl Cancer Inst 107(7). https://doi.org/10.1093/jnci/djv135
Chava S, Reynolds CP, Pathania AS, Gorantla S, Poluektova LY, Coulter DW et al (2020) miR-15a-5p, miR-15b-5p, and miR-16-5p inhibit tumor progression by directly targeting MYCN in neuroblastoma. Mol Oncol 14(1):180–196. https://doi.org/10.1002/1878-0261.12588
Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM et al (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27(2):289–297. https://doi.org/10.1200/JCO.2008.16.6785
Colletti M, Petretto A, Galardi A, Di Paolo V, Tomao L, Lavarello C et al (2017) Proteomic analysis of neuroblastoma-derived exosomes: new insights into a metastatic signature. Proteomics 17(23–24). https://doi.org/10.1002/pmic.201600430
Colon NC, Chung DH (2011) Neuroblastoma. Adv Pediatr Infect Dis 58(1):297–311. https://doi.org/10.1016/j.yapd.2011.03.011
Daudigeos-Dubus E, Led L, Rouffiac V, Bawa O, Leguerney I, Opolon P et al (2014) Establishment and characterization of new orthotopic and metastatic neuroblastoma models. In Vivo 28(4):425–434
Dorronsoro A, Robbins PD (2013) Regenerating the injured kidney with human umbilical cord mesenchymal stem cell-derived exosomes. Stem Cell Res Ther 4(2):39. https://doi.org/10.1186/scrt187
Fonseka P, Liem M, Ozcitti C, Adda CG, Ang CS, Mathivanan S (2019) Exosomes from N-Myc amplified neuroblastoma cells induce migration and confer chemoresistance to non-N-Myc amplified cells: implications of intra-tumour heterogeneity. J Extracell Vesicles 8(1):1597614. https://doi.org/10.1080/20013078.2019.1597614
Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ (1999) Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94(11):3791–3799
Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75(2):193–208. https://doi.org/10.1007/s00018-017-2595-9
Huang M, Weiss WA (2013) Neuroblastoma and MYCN. Cold Spring Harb Perspect Med 3(10):a014415. https://doi.org/10.1101/cshperspect.a014415
Kalani A, Tyagi N (2015) Exosomes in neurological disease, neuroprotection, repair and therapeutics: problems and perspectives. Neural Regen Res 10(10):1565–1567. https://doi.org/10.4103/1673-5374.165305
Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS et al (2010) Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 4(3):214–222. https://doi.org/10.1016/j.scr.2009.12.003
Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G et al (2012) Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126(22):2601–2611. https://doi.org/10.1161/CIRCULATIONAHA.112.114173
Legler JM, Ries LA, Smith MA, Warren JL, Heineman EF, Kaplan RS et al (1999) Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst 91(16):1382–1390. https://doi.org/10.1093/jnci/91.16.1382
London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H et al (2005) Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children’s Oncology Group. J Clin Oncol 23(27):6459–6465. https://doi.org/10.1200/JCO.2005.05.571
Ma J, Xu M, Yin M, Hong J, Chen H, Gao Y et al (2019) Exosomal hsa-miR199a-3p promotes proliferation and migration in neuroblastoma. Front Oncol 9:459. https://doi.org/10.3389/fonc.2019.00459
Maas SLN, Breakefield XO, Weaver AM (2017) Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol 27(3):172–188. https://doi.org/10.1016/j.tcb.2016.11.003
Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B (2018) Exosome-based cell-cell communication in the tumor microenvironment. Front Cell Dev Biol 6:18. https://doi.org/10.3389/fcell.2018.00018
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362(23):2202–2211. https://doi.org/10.1056/NEJMra0804577
Nakata R, Shimada H, Fernandez GE, Fanter R, Fabbri M, Malvar J et al (2017) Contribution of neuroblastoma-derived exosomes to the production of pro-tumorigenic signals by bone marrow mesenchymal stromal cells. J Extracell Vesicles. 6(1):1332941. https://doi.org/10.1080/20013078.2017.1332941
Neviani P, Wise PM, Murtadha M, Liu CW, Wu CH, Jong AY et al (2019) Natural killer-derived Exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res 79(6):1151–1164. https://doi.org/10.1158/0008-5472.CAN-18-0779
Park JR, Eggert A, Caron H (2008) Neuroblastoma: biology, prognosis, and treatment. Pediatr Clin N Am 55(1):97–120, x. https://doi.org/10.1016/j.pcl.2007.10.014
Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF et al (2015) Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol 33(27):3008–3017. https://doi.org/10.1200/JCO.2014.59.4648
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383. https://doi.org/10.1083/jcb.201211138
Schmidt O, Teis D (2012) The ESCRT machinery. Curr Biol 22(4):R116–R120. https://doi.org/10.1016/j.cub.2012.01.028
Seeger RC (2011) Immunology and immunotherapy of neuroblastoma. Semin Cancer Biol 21(4):229–237. https://doi.org/10.1016/j.semcancer.2011.09.012
Speleman F, Park JR, Henderson TO (2016) Neuroblastoma: a tough nut to crack. Am Soc Clin Oncol Educ Book 35:e548–e557. https://doi.org/10.14694/EDBK_159169. https://doi.org/10.1200/EDBK_159169
Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5(3):219–234. https://doi.org/10.1038/nrd1984
Willis GR, Kourembanas S, Mitsialis SA (2017) Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med 4:63. https://doi.org/10.3389/fcvm.2017.00063
Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X et al (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30(7):1556–1564. https://doi.org/10.1002/stem.1129
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX et al (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363(14):1324–1334. https://doi.org/10.1056/NEJMoa0911123
Zhang G, Wang D, Miao S, Zou X, Liu G, Zhu Y (2016) Extracellular vesicles derived from mesenchymal stromal cells may possess increased therapeutic potential for acute kidney injury compared with conditioned medium in rodent models: A meta-analysis. Exp Ther Med 11(4):1519–1525. https://doi.org/10.3892/etm.2016.3076
Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A et al (2015) Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg 122(4):856–867. https://doi.org/10.3171/2014.11.JNS14770
Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19. https://doi.org/10.1186/s13578-019-0282-2
Acknowledgments
Dr. Challagundla’s laboratory is supported in whole or part from the NIH/NCI grant (K22CA197074-01); Leukemia Research Foundation (LRF) grant, the Nebraska State DHHS (LB506); UNMC Pediatric Cancer Research Center; Fred and Pamela Buffett Cancer Center’s pilot grant (P30 CA036727) in conjunction with the UNMC Pediatric Cancer Research Center; and the Department of Biochemistry and Molecular Biology start-up. Heather Richard and Arya Pokhrel are thankful to Terri L. Gulick, Jaynie E. Bird, Michele Merrill, and Heidi N. Kaschke and acknowledge the support of the UNMC High School Alliance Health Sciences Enrichment Program.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Richard, H., Pokhrel, A., Chava, S., Pathania, A., Katta, S.S., Challagundla, K.B. (2020). Exosomes: Novel Players of Therapy Resistance in Neuroblastoma. In: Birbrair, A. (eds) Tumor Microenvironment . Advances in Experimental Medicine and Biology, vol 1277. Springer, Cham. https://doi.org/10.1007/978-3-030-50224-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-50224-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50223-2
Online ISBN: 978-3-030-50224-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)