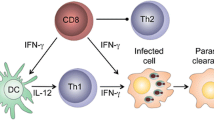
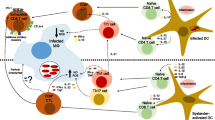
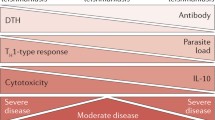
Abstract
Leishmaniasis is a major public health problem in several areas of the world, occurring in large areas of the Middle East, Africa, and Latin America. The Leishmania complex is a group of closely related parasites that occupy a wide variety of ecologic niches and cause a spectrum of clinical disease. Infection is initiated by the bite of a sandfly, which injects the motile form, called promastigotes. Once inside the mammalian host, Leishmania are obligatory intramacrophage parasites, multiplying in these cells as nonmotile amastigotes. Basic biologic aspects of the parasite are thoroughly reviewed elsewhere (1,2).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Peters W, Killick-Kendrick R (eds): The Leishmaniasis in Biology and Medicine. Vol. II. Clinical Aspects and Control. Academic Press, Orlando, FL, 1987.
Chang KP, Bray RS (eds): Human Parasitic Diseases. Vol. 1. Leishmaniasis. Elsevier Science Publishers, New York, 1985.
Manson-Bahr PEC: Active immunization in leishmaniasis. In Garnham PCC, Pierce AE, Ratt I (eds): Immunity to Protozoa. Blackwell Scientific, Oxford, 1963, p. 246.
Montenegro J: A cutis-reaccao na leishmaniose. Ann Fac Med Univ Sao Paulo 1926; 1:323.
Reed SG, Badaro R, Masur H, et al: Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg 1986;35:79.
Badaro R, Pedral-Sampaio D, Johnson WD Jr, Reed SG: Evaluation of the stability of a soluble intradermal skin test antigen preparation in American visceral leishmaniasis. Trans R Soc Trop Med Hyg 1990;84:226.
Castes M, Agnelli A, Verde O, Rondon AJ: Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Immunol Immunopathol 1983;27:176.
Carvalho EM, Johnson WD, Barreto E, et al: Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol 1985; 135:4144.
Manson-Bahr PEC: The leishmanin test and immunity in Kala-azar. E Afr Med J 1961;38:165.
Manson-Bahr PEC, Heisch RB, Garnham PCC: Studies in leishmaniasis in East Africa: the Montenegro test in Kala-azar in Kenya. Trans R Soc Trop Med Hyg 1959;53:380.
Andrade TM, Teixeira R, Andrade JAF, et al: Estudo da hipersensibili-dade de tipo retardado na leishmaniose visceral. Rev Inst Med Trop Sao Paulo 1982;24:298.
Badaro R, Jones TC, Carvalho EM, et al: New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis 1986; 154:1003.
Mayrink W, Williams P, Costa CA, et al: An experimental vaccine against American dermal leishmaniasis: experience in the state of Espirito Santo. Ann Trop Med Parasitol 1985;79:259.
Carvalho EM, Teixeira RS, Johnson Jr WD: Cell mediated immunity in American visceral leishmaniasis. Infect Immun 1981;48:409.
Carvalho EM, Badaro R, Reed SG, et al: Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest 1985; 76:2066.
Sacks DL, Lata Lal S, Shrivastava SN, et al: An analysis of T cell responsiveness in Indian kala-azar. J Immunol 1987; 138:908.
Ciliari E, Liew FY, Lo Campo P, et al: Suppression of IL-2 production by cryopreserved peripheral blood mononuclear cells from patients with active visceral leishmaniasis in Sicily. J Immunol 1988; 140:2721.
Wyler DJ: Circulating factor from a Kala-azar patient suppresses in vitro anti-leishmanial T cell proliferation. Trans R Soc Trop Med Hyg 1982,76:304.
Barral A, Carvalho EM, Badaro R, Barral-Netto M: Suppression of lymphocyte proliferative responses by sera from patients with American visceral leishmaniasis. Am J Trop Med Hyg 1986;35:735.
Carvalho EM, Bacellar O, Barral A, et al: Antigen-specific immunosuppression in visceral leishmaniasis is T cell mediated. J Clin Invest 1989;83:860.
Ridley DS, Ridley MJ: The evolution of the lesion in cutaneous leishmaniasis. Int J Dermatol 1983; 18:50.
Barral A, Jesus A, Almeida RP, et al: Evaluation of T cell subsets in the lesion infiltrates of human cutaneous and mucocutaneous leishmaniasis. Parasite Immunol 1987;9:487.
Pirmez C, Cooper C, Paes-Oliveira M, et al: Immunologic responsiveness in American cutaneous leishmaniasis lesions. J Immunol 1990; 145:3100.
Nathan CF, Murray HW, Wiebe ME, Rubin BY: Identification of interferon gamma as the lymphokine that activates human macrophages oxidative metabolism and antimicrobial activity. J Exp Med 1983; 158: 670.
Murray HW, Rubin BY, Rothermel CD: Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocyte: evidence that interferon gamma is the activating lymphokine. J Clin Invest 1983;72:1506.
Weiser WY, Van Neil A, Clark SC, et al: Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med 1987; 166:1436.
Corradin SB, Mauel J: Phagocytosis of Leishmania enhances macrophage activation by IFN-γ and lipopolysaccharide. J Immunol 1991; 146:279.
Green SJ, Meltzer MS, Hibbs JB, Nacy CA: Activated macrophage destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol 1990; 144:278.
Mosmann TR, Cherwinski H, Bond MW, et al: Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986; 136:2348.
Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR: Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays and monoclonal antibodies. J Exp Med 1987; 166:1229.
Scott P, Natovitz P, Coffman R, Pearce E, Sher. A. Immunoregulation of cutaneous leishmaniasis: T cell lines that transfer protective immunity or exacerbation belong to different T-helper subsets and respond to distinct parasite antigens. J Exp Med 1988; 168:1675.
Heinzel FP, Sadick MD, Holaday BJ, et al: Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis: evidence for expansion of distinct helper T cell subsets. J Exp Med 1989; 169:59.
De Libero G, Kaufmann SHE: Antigen-specific Lyt2+ cytolytic T lymphocytes from mice infected with the intracellular bacterium Listeria monocytogenes. J Immunol 1986;137:2688–2694.
Weiss WR, Sedegah M, Beaudoin RL, et al: CD8+ T cells (cytotoxic/suppressors) are required for protection in mice immunized with malaria sporozoites. Proc Natl Acad Sci USA 1988;85:573–576.
Melby PC, Neva FA, Sacks DL: Profile of human T cell response to leishmanial antigens: analysis by immunoblotting. J Clin Invest 1989; 83:1868–1875.
Melby PC, Sacks DL: Identification of antigens recognized by T cells in human leishmaniasis: analysis of T cell clones by immunoblotting. Infect Immun 1989;57:2971.
Reed SG, Carvalho EM, Sherbert CH, et al: In vitro response to Leishmania antigens by lymphocytes from patients with leishmaniasis or Chagas’ disease. J Clin Invest 1990; 85:690.
Russo DM, Barral-Netto M, Armitage RJ, et al: Identification of circulating γδ T cells in leishmaniasis patients. Submitted.
Brenner MB, McLean J, Dialynas DP, et al: Identification of a putative second T-cell receptor. Nature 1986;322:145.
Bank I, DePinho RA, Brenner MB, et al: A functional T3 molecule associated with a novel heterodimer on the surface of immature human thymocytes. Nature 1986;322:79.
Brenner MB, Strominger JL, Krangel MS: The gamma-delta T cell receptor. Adv Immunol 1988;43:133.
Lew AM, Pardoll DM, Maloy WL, et al: Characterization of T cell receptor gamma chain expression in a subset of murine thymocytes. Science 1986;334:1401.
Stingl G, Gunter KT, Tschachler E, et al: Thy-1+ dendritic epidermal cells belong to the T cell lineage. Proc Natl Acad Sci USA 1987;84:2430.
Goodman T, Lefrancois L: Expression of the gamma-delta T cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature 1988;333:855.
Palliard X, Yssel H, Basnnchard D, et al: Antigen specific and MHC nonrestricted cytotoxicity of T cell receptor αβ and γδ human T cell clones isolated in IL-4. J Immunol 1989;143:452.
Kozbor D, Trinchieri G, Monos, DS, et al: Human TCR-γ+/δ+, CD8+ T lymphocytes recognize tetanus toxoid in an MHC-restricted fashion. J Exp Med (1989);169:1847.
Seki S, Abo T, Masuda, T, et al: Identification of activated T cell receptor γδ lymphocytes in the liver of tumor-bearing hosts. J Clin Invest 1990;86:409.
Margolick JB, Scott, ER, Odaka N, Saah. A: Flow cytometric analysis of γδ T cells and natural killer cells in HIV-1 infection. Clin Immunol Im-munopathol 1991; 58:126.
Haas W, Kaufmann S, Martinez-A C: The development and function of γδ T cells. In Gallagher R (ed): Immunology Today. Vol. 11. Elsevier Trends Journals, Cambridge, 1990, P, 340.
Roussilhon C, Agrapart M, Ballet JJ, Bensussan A: T lymphocytes bearing the γδ T cell receptor in patients with acute Plasmodium falciparum malaria. J Infect Dis 1990; 162:283.
Ho M, Webster HK, Tongtawe P, et al: Increased γδ T cells in acute Plasmodium falciparum malaria. Immunol Lett 1990;25:139.
Modlin RL, Pirmez C, Hofman F, et al: Lymphocytes bearing antigen specific γδ T-cell receptors accumulate in human infectious disease lesions. Nature 1989;339:544.
Haregewoin A, Soman G, Hom RC, Finberg RW: Human γδ+ T cells respond to mycobacterial heat-shock protein. Nature 1989;340:309.
Kabelitz D, Bender A, Schondelmaier S, et al: A large fraction of human peripheral blood γδ T cells is activated by Mycobacterium tuberculosis but not by its 65 kd heat shock protein J Exp Med 1990; 171:667.
Holoshitz J, Koning F, Coligan J, et al: Isolation of CD4- CD8+ mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature 1989;339:226.
Balbi B, Moller DR, Kirby M, et al: Increased numbers of T lymphocytes with γδ-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J Clin Invest 1990;85:1353.
Burns Jr JM, Scott JM, Russo DM, et al: Characterization of a membrane antigen of Leishmania amazonensis which stimulates human immune responses. J Immunol 1990; 146:742.
Born W, Hafpp M, Dallas A, et al: Recognition of heat shock proteins and γδ cell function. Immunol Today 1990;11(2):40.
Searle S, Campos, AJ, Coulson RM, et al: A family of heat shock protein 70-related genes are expressed in the promastigotes of Leishmania major. Nucleic Acids Res 1989; 17:5081.
MacFarlane J, Blaxter ML, Bishop RP, et al: Identification and characterization of a Leishmania donovani antigen belonging to the 70-kDa heat-shock protein family. Eur J Biochem 1990; 190:377.
Colmer-Gould V, Quintao, LG, Keithly J, Nogueira N: A common major surface antigen on amastigotes and promastigotes of Leishmania species. J Exp Med 1985; 162:902.
Reed SG, Badaro R, Lloyd RMC: Identification of specific and cross-reactive antigens of Leishmania donovani chagasi by human infection sera. J Immunol 1987; 138:1596.
Russell DG, Wilheim H: The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol 1986; 138:1596.
Russell DG, Alexander J: Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol 1988; 140:1274.
Nascimento E, Mayrink W, da Costa CA, et al: Vaccination of humans against cutaneous leishmaniasis: cellular and humoral immune responses. Infect Immun 1990;58:2198.
Button LL, Reiner NE, McMaster WR. Modification of Leishmania gp63 genes by the polymerase chain reaction for expression of nonfusion protein at high levels in Escherichia coli: application to mapping protective T cell determinants. Mol. Biochem. Parasitol. 1991;44:213.
Russo DM, Burns JM, Carvalho E, et al: Human T cell responses to gp63, a surface antigen of Leishmania. J. Immunol. 1991;147:3575.
Jardim A, Alexander J, Teh HS, et al: Immunoprotective Leishmania major synthetic T cell epitopes. J Exp, Med (1990). 172:645.
Anderson S, David JR, McMahon-Pratt D: In vivo protection against Leishmania mexicana mediated by monoclonal antibodies. J Immunol 1983;131:1616.
Champsi J, McMahon-Pratt D: Membrane glycoprotein M-2 protects against Leishmania amazonensis infection. Infect Immun 1988; 52:3272.
Handman E, Mitchell GF: Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Nat Acad Sci USA 1985;82:5910.
McNeely TB, Turco SJ: Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. J Immunol 1990; 144:2745.
Moll H, Mitchell GF, McConville MJ, Handman E: Evidence for T cell recognition in mice of a purified lipophosphoglycan from Leishmania major. Infect Immun 1989;57:3349.
Mendonca SCF, Russel DG, Coutinho SG: Analysis of the human T cell responsiveness to purified antigens of Leishmania: Lipophosphoglycan (LPG) and glycoprotein 63 (gp 63). Clin. Exp. Immonol 1991;83:472.
Russo DM, Turco SJ, Burns JM Jr. and Reed SG. 1992. Stimulation of human T lymphocytcs by Leishmania Lipophosphoglycan-associated proteins. J. Immunol 148:202.
Crawford GD, Wyler DJ, Dinarello CA: Parasite-monocyte interactions in human leishmaniasis: production of interleukin-1 in vitro. J Infect Dis 1985;152:315.
Staruch MJ, Wood DD: The adjuvanticity of interleukin-1 in vivo. J Immunol 1983;130:2191.
Akuffo H, Kaplan G, Kiessling R, et al: Administration of recombinant interleukin-2 reduces the local parasite load of patients with disseminated cutaneous leishmaniasis. J Infect Dis 1990; 161:775.
Feng ZY, Louis J, Kindler VT, et al: Aggravation of experimental cutaneous leishmaniasis in mice by administration of interleukin 3. Eur J Immunol 1988; 18:1245.
Lelchuk R, Graveley, R, Liew FY: Susceptibility to murine cutaneous leishmaniasis correlates with the capacity to generate interleukin 3 in response to Leishmania antigen in vitro. Cell Immunol 1988; 111:66.
Ho JL, Reed SG, Wick EA, Giordano M: Granulocyte-macrophage and macrophage colony stimulating factors activate intramacrophage killing of Leishmania mexicana amazonensis. J Infect Dis 1990; 162:224.
Griel J, Bodendorfer B, Rollinghoff M, Solbach W: Application of recombinant granulocyte-macrophage colony stimulating factor has a detrimental effect in experimental murine leishmaniasis. Eur J Immunol 1988;18:1525.
Corcoran LM, Metcalf D, Edwards SJ, Handman E: GM-CSF produced by recombinant vaccinia virus or in GM-CSF transgenic mice has no effect in vivo on murine cutaneous leishmaniasis. J Parasitol 1988; 74:763.
Hoover DL, Nacy CA, Meltzer MS: Human monocyte activation for cytotoxicity against intracellular L. donovani amastigotes induction of microbicidal activity by interferon gamma. Cell Immunol 1985;94:500.
Sadick MD, Heinzel FP, Holaday BJ, et al: Cure of murine leishmaniasis with anti-interleukin-4 monoclonal antibody: evidence for a T cell-dependent interferon-gamma independent mechanism. J Exp Med 1990;171:115.
Murray, HW, Stein J, Welte K: Experimental visceral leishmaniasis: production of interleukin-2 and gamma interferon, tissue immune reactions, and response to treatment with interleukin-2 and gamma interferon. J Immunol 1987; 138:2290.
Reed SG, Barral-Netto M, Inverso JA: Treatment of experimental visceral leishmaniasis with lymphokine encapsulated in liposomes. J Immunol 1984;132:3116.
Murray HW, Berman JD, Wright SD: Immunochemotherapy for intracellular Leishmania donovani infection: gamma interferon plus pen-tavalent antimony. J Infect Dis 1988; 157:973.
Harms G, Chehadi AK, Racz P, et al: Effects of intradermal gamma-interferon in cutaneous leishmaniasis. Lancet 1989; 10:1287.
Badaro R, Falcoff E, Badaro FS, et al: Treatment of visceral leishmaniasis with pentavalent antimony and interferon gamma. N Engl J Med 1990;322:16.
Scuderi P, Lam KS, Ryan KJ, et al: Raised serum levels of tumor necrosis factor in parasitic infections. Lancet 1986;2:1364.
Barral-Netto M, Badaro R, Banal A, et al: Tumor necrosis factor Cachectin in human visceral leishmaniasis. J Infect Dis (in press).
Titus RG, Sherry B, Cerami A: Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med 1989;6:2097.
Liew FY, Parkinson C, Millott S, et al: Tumor necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunobiology 1990;69:570.
Reed SG, Grabstein KH, Pihl D, Morrisey PJ: Recombinant granulocyte-macrophage colony-stimulating factor restores deficient immune responses in mice with chronic Trypanosoma cruzi infections. J Immunol 1990; 145:1564.
Tarleton RL, Kuhn RE: Restoration of in vitro immune responses of spleen cells from mice infected with Trypanosoma cruzi by super-natants containing interleukin 2. J Immunol 1984; 133:1570.
Reed SG, Inverso JA, Roters SB: Suppressed antibody responses to sheep erythrocytes in mice with chronic Trypanosoma cruzi infections are re stored with interleukin-2. J Immunol 1984; 133:3333.
Reed SG, Pihl DL, Grabstein KH: Immune deficiency in chronic Trypanosoma cruzi infection: recombinant interleukin-1 restores Th function for antibody production. J Immunol 1989; 142:2067.
DeTitto EH, Catterall JR, Remington JS: Activity of recombinant tumor necrosis factor on Toxoplasma gondii and Trypanosoma cruzi. J Immunol 1985; 157:1342.
Grabstein KH, Urdal DL, Tushinski RJ, et al: Induction of macrophage tumorcidal activity by granulocyte-macrophage colony-stimulating factor. Science 1986;232:506.
Lotze MT, Matory YL, Ettinghansen SE, et al: In vivo administration of purified human interleukin-2. II. Half-life immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL-2. J Immunol 1985; 135:2865.
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1993 Springer-Verlag New York, Inc.
About this chapter
Cite this chapter
Russo, D.M., Barral-Netto, M., Barral, A., Reed, S.G. (1993). Human T-Cell Responses in Leishmania Infections. In: Sun, T. (eds) Progress in Clinical Parasitology. Springer, New York, NY. https://doi.org/10.1007/978-1-4612-2732-8_5
Download citation
DOI: https://doi.org/10.1007/978-1-4612-2732-8_5
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4612-7646-3
Online ISBN: 978-1-4612-2732-8
eBook Packages: Springer Book Archive