Abstract
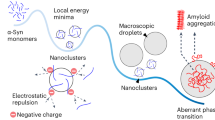
Liquid-liquid phase separation (LLPS) has emerged as an important phenomenon associated with formation of membraneless organelles. Recently, LLPS has been shown to act as nucleation centers for disease-associated protein aggregation and amyloid fibril formation. Phase-separated α-synuclein droplets gradually rigidify during the course of protein aggregation, and it is very challenging to understand the biomolecular interactions that lead to liquid-like to solid-like transition using conventional ensemble measurements. Here, we describe a spectrally-resolved fluorescence microscopy based Förster resonance energy transfer (FRET) imaging to probe interactions of α-synuclein in individual droplets during LLPS-mediated aggregation. By acquiring entire emission spectral profiles of individual droplets upon sequential excitation of acceptors and donors therein, this technique allows for the quantification of sensitized emission proportional to the extent of FRET, which enables interrogation of the evolution of local interactions of donor-/acceptor-labeled α-synuclein molecules within each droplet. The present study on single droplets is not only an important development for studying LLPS but can also be used to investigate self-assembly or aggregation in biomolecular systems and soft materials.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Banani SF, Rice AM, Peeples WB et al (2016) Compositional control of phase-separated cellular bodies. Cell 166:651–663
Shin Y, Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease. Science 357:eaaf4382
Riback JA, Katanski CD, Kear-Scott JL et al (2017) Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168:1028–1040.e19
Banani SF, Lee HO, Hyman AA et al (2017) Biomolecular condensates: Organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18:285–298
Brangwynne CP, Eckmann CR, Courson DS et al (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324:1729–1732
Hyman AA, Weber CA, Jülicher F (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30:39–58
Boeynaems S, Alberti S, Fawzi NL et al (2018) Protein phase separation: a new phase in cell biology. Trends Cell Biol 28:420–435
Alberti S (2017) Phase separation in biology. Curr Biol 27:R1097–R1102
Holehouse AS, Pappu RV (2018) Functional implications of intracellular phase transitions. Biochemistry 57:2415–2423
Feric M, Vaidya N, Harmon TS et al (2016) Coexisting liquid phases underlie nucleolar subcompartments. Cell 165:1686–1697
Kaiser TE, Intine RV, Dundr M (2008) De novo formation of a subnuclear body. Science 322:1713–1717
Lin Y, Protter DSW, Rosen MK et al (2015) Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol Cell 60:208–219
Brangwynne CP, Tompa P, Pappu RV (2015) Polymer physics of intracellular phase transitions. Nat Phys 11:899–904
Wei MT, Elbaum-Garfinkle S, Holehouse AS et al (2017) Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat Chem 9:1118–1125
Molliex A, Temirov J, Lee J et al (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163:123–133
Nott TJ, Petsalaki E, Farber P et al (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell 57:936–947
Zhang H, Elbaum-Garfinkle S, Langdon EM et al (2015) RNA controls polyQ protein phase transitions. Mol Cell 60:220–230
Vecchi G, Sormanni P, Mannini B et al (2020) Proteome-wide observation of the phenomenon of life on the edge of solubility. Proc Natl Acad Sci USA 117:1015–1020
Boke E, Ruer M, Wühr M et al (2016) Amyloid-like self-assembly of a cellular compartment. Cell 166:637–650
Murakami T, Qamar S, Lin JQ et al (2015) ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88:678–690
Patel A, Lee HO, Jawerth L et al (2015) A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162:1066–1077
Wegmann S, Eftekharzadeh B, Tepper K et al (2018) Tau protein liquid–liquid phase separation can initiate tau aggregation. EMBO J 37:e98049
Ambadipudi S, Biernat J, Riedel D et al (2017) Liquid-liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat Commun 8:275
Conicella AE, Zerze GH, Mittal J et al (2016) ALS mutations disrupt phase separation mediated by α-helical structure in the TDp-43 low-complexity c-terminal domain. Structure 24:1537–1549
Ray S, Singh N, Kumar R et al (2020) α-Synuclein aggregation nucleates through liquid–liquid phase separation. Nat Chem 12:705–716
Goldman RD, Spector DL, Masters BR (2006) Live cell imaging, a laboratory manual. J Biomed Opt 11:019901
Bolte S, Cordelières FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232
Hu K, Johnson J, Florens L, Fraunholz M et al (2006) Cytoskeletal components of an invasion machine – the apical complex of Toxoplasma gondii. PLoS Pathog 2:0121–0138
Kumar P, Lyle KS, Gierke S et al (2009) GSK33 phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J Cell Biol 184:895–908
Waters JC (2009) Accuracy and precision in quantitative fluorescence microscopy. J Cell Biol 185:1135–1148
Gordon GW, Berry G, Liang XH et al (1998) Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy. Biophys J 74:2702–2713
Zeug A, Woehler A, Neher E, Ponimaskin EG (2012) Quantitative intensity-based FRET approaches – a comparative snapshot. Biophys J 103:1821–1827
Pentcheva T, Edidin M (2001) Clustering of peptide-loaded MHC class I molecules for endoplasmic reticulum export imaged by fluorescence resonance energy transfer. J Immunol 166:6625–6632
Andrews DL (1989) A unified theory of radiative and radiationless molecular energy transfer. Chem Phys 135:195–201
Sekar RB, Periasamy A (2003) Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J Cell Biol 160:629–633
Lakowicz JR (2006) Principles of fluorescence spectroscopy. Springer, US, Boston, MA
Kenworthy AK (2001) Imaging protein-protein interactions using fluorescence resonance energy transfer microscopy. Methods 24:289–296
Periasamy A (2001) Fluorescence resonance energy transfer microscopy: a mini review. J Biomed Opt 6:287
Lemke EA, Deniz AA (2011) Förster resonance energy transfer. In: Comprehensive nanoscience and technology. Elsevier, pp 127–151
Berney C, Danuser G (2003) FRET or no FRET: a quantitative comparison. Biophys J 84:3992–4010
Kapanidis AN, Laurence TA, Nam KL et al (2005) Alternating-laser excitation of single molecules. Acc Chem Res 38:523–533
Das S, Sharma DK, Chakrabarty S et al (2015) Bioactive polymersomes self-assembled from amphiphilic PPO-glyco polypeptides: Synthesis, characterization, and dual-dye encapsulation. Langmuir 31:3402–3412
Sarkar A, Behera T, Sasmal R et al (2020) Cooperative supramolecular block copolymerization for the synthesis of functional axial organic heterostructures. J Am Chem Soc 142:11528–11539
Bates M, Jones SA, Zhuang X (2013) Stochastic optical reconstruction microscopy (STORM): a method for superresolution fluorescence imaging. Cold Spring Harb Protoc 8:498–520
Möckl L, Lamb DC, Bräuchle C (2014) Super-resolved fluorescence microscopy: Nobel prize in chemistry 2014 for Eric Betzig, Stefan Hell, and William E. Moerner. Angew Chemie Int Ed Engl 53:13972–13977
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Mahato, J., Ray, S., Maji, S.K., Chowdhury, A. (2023). Spectrally Resolved FRET Microscopy of α-Synuclein Phase-Separated Liquid Droplets. In: Cieplak, A.S. (eds) Protein Aggregation. Methods in Molecular Biology, vol 2551. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2597-2_27
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2597-2_27
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2596-5
Online ISBN: 978-1-0716-2597-2
eBook Packages: Springer Protocols