Abstract
With a surprisingly complex genome and an ever-expanding genetic toolkit, the sea anemone Nematostella vectensis has become a powerful model system for the study of both development and whole-body regeneration. Here we provide the most current protocols for short-hairpin RNA (shRNA )-mediated gene knockdown and CRISPR/Cas9-targeted mutagenesis in this system. We further show that a simple Klenow reaction followed by in vitro transcription allows for the production of gene-specific shRNAs and single guide RNAs (sgRNAs) in a fast, affordable, and readily scalable manner. Together, shRNA knockdown and CRISPR/Cas9-targeted mutagenesis allow for rapid screens of gene function as well as the production of stable mutant lines that enable functional genetic analysis throughout the Nematostella life cycle.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
- Nematostella vectensis
- Cnidaria
- CRISPR
- shRNA
- Mutagenesis
- Electroporation
- Knockdown
- Cas9
- Microinjection
- Genome editing
1 Introduction
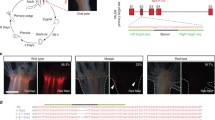
As a member of phylum Cnidaria, the sister group to Bilateria, the starlet sea anemone Nematostella vectensis is an important model for the study of animal development and evolution (Fig. 1) [1,2,3,4]. More recently, Nematostella has also become a popular system for the study of whole-body regeneration [5,6,7]. Nematostella polyps are highly regenerative, capable of replacing all missing parts through cellular proliferation in approximately 1 week (Fig. 2) [8, 9]. The presence of similar capabilities throughout most cnidarian species [10] makes whole-body regeneration a potentially shared characteristic of basal metazoans and uniquely positions Nematostella as a model to study the molecular and genetic basis for ancient and conserved mechanisms of regeneration.
Life cycle of Nematostella vectensis . The lifecycle of Nematostella vectensis is an example of indirect development . Eggs divide rapidly following fertilization and gastrulate to set up the germ layers of the animals and form a transient stage called a gastrula. The planula stage comes next. The planula is a motile larva that moves due to a ciliary organ called the apical tuft. After a few days, the planula undergoes metamorphosis to become a primary polyp. Growth from feeding causes the primary polyp to grow to a sexually mature adult polyp. Both primary and adult polyps are capable of whole-body regeneration. Currently, the egg and blastomeres formed by early mitotic cleavages are the most readily available stages for genetic manipulation
Regeneration in Nematostella vectensis . (a) An uninjured Nematostella vectensis polyp. (b) Time course of oral regeneration after amputation aboral to the pharynx. All missing tissue is restored by 6 days of regeneration (6 dR). Tentacle growth continues for approximately an additional week (12 dR). Scale bar – 400 μm
Gene-specific knockdown by shRNA was only recently reported in Nematostella [11]. The Cnidarian microRNA pathway most commonly regulates target mRNAs by cleavage, similar to the mechanism of small interfering RNAs and allows for highly specific knockdown of individual genes [11,12,13]. For shRNA design, a free web-based interface or downloadable programs support algorithms that can be used to design gene-specific 19-mer targeting motifs. Optimal targeting motifs are then incorporated into a standardized oligo design that allows for cost-effective DNA template production and in vitro transcription of shRNAs by T7 RNA polymerase (Fig. 3a, a′). Additionally, DNA templates for shRNA production can be produced from standard commercially produced oligonucleotides making it cost effective. Once produced, shRNA can be delivered either by microinjection or electroporation of eggs [11, 14]. The method we describe produces micrograms of shRNA per in vitro transcription (IVT), making each reaction suitable for hundreds of microinjections or multiple electroporation experiments.
Design and production of shRNAs and sgRNAs. (a) Schematic of universal shRNA and gene-specific shRNA oligonucleotides design. (a′) Graphic outline of shRNA production protocol . (b) Schematic of universal sgRNA and target-specific sgRNA oligonucleotides designed using CRISPRscan [14]. (b′) Graphic outline of sgRNA production protocol
Initial genome editing efforts in Nematostella used TALENs (Transcription Activator-Like Effector Nucleases) or multistep, cloning-based methods for CRISPR sgRNA production [15]. We have recently adopted a method for sgRNA production leveraging sgRNA design using CRISPRscan [16, 17] followed by T7-mediated transcription from a DNA template produced by a one-step Klenow extension reaction (Fig. 3b, b′). As with shRNA , this method produces micrograms of sgRNA molecules that can be used for hundreds of microinjection experiments. While the current preferred method for targeted mutagenesis is the injection of preloaded ribonucleoproteins (RNPs) of sgRNA and Cas9 protein, similar mutation rates can be achieved using a Nematostella codon-optimized Cas9 mRNA and slightly lower mutation rates can be obtained with a zebrafish codon-optimized Cas9 mRNA [18].
Although current methods for the introduction of functional RNA molecules are limited to early embryogenesis (Fig. 1), the future is bright for functional genetic analysis restricted only to polyp stage animals. A functional heat shock promoter [16] as well as the increased efficacy of genetic insertions by homology-directed repair [16, 19] will likely soon lead to the establishment of transgenic lines containing spatially and temporally controlled genetic tools, such as inducible site-specific DNA recombinase systems (e.g., Cre:Lox and FLP:FRT) or other synthetic conditional alleles found in traditional model organisms [20,21,22,23,24]. Continued development of the Nematostella toolkit will not only allow the functional interrogation of the genetic requirements of whole-body regeneration but will also enable the direct comparison of embryogenesis and regeneration in the same animal.
Here, we discuss current strategies for functional genetic analysis by shRNA-mediated knockdown and CRISPR/Cas9-targeted mutagenesis in Nematostella. Both the shRNA knockdown and CRISPR/Cas9-targeted mutagenesis protocols detailed here have four main steps: (1) Design; (2) Production; (3) Delivery; and (4) Screening and Care. While the general methods for these two protocols are similar, their purposes are complementary and allow for genetic analysis throughout the life cycle of the animal.
2 Materials
All solutions should be prepared with molecular biology grade and nuclease-free reagents unless otherwise stated.
2.1 shRNA Production
-
1.
shRNA candidate sequence prediction algorithm or web-based tool (see Note 1).
-
2.
shRNA gene-specific reverse strand oligonucleotide with the following structure:
-
5′-AA-[19 bp shRNA candidate sequence]-TCTCTTGAA-[reverse complement candidate sequence]-TATAGTGAGT-3′ (Fig. 3a).
-
-
3.
Non-targeting shRNA oligonucleotide.
-
4.
Universal shRNA forward oligonucleotide with T7 promoter sequence:
-
5′-TAATACGACTCACTATA-3′.
-
-
5.
N-terminal truncated DNA polymerase I (e.g., Klenow fragment).
-
6.
10× DNA polymerase buffer: 50 mM NaCl, 10 mM Tris–HCl, 10 mM MgCl2, 1 mM dithiothreitol (DTT), pH 7.9 (see Note 2).
-
7.
10 mM dNTP mixture.
-
8.
High-yield T7 in vitro transcription kit (e.g., AmpliScribe™ T7-Flash™ transcription kit, Lucigen).
-
9.
RNA purification kit.
-
10.
shRNA annealing mix solution: 3 μL 100 μM universal shRNA forward oligonucleotide, 4 μL nuclease-free water.
-
11.
Template extension reaction: 2 μL 10× DNA polymerase buffer, 1 μL 10 mM dNTP, 1 μL N-terminal truncated DNA polymerase I (5000 U/mL), 6 μL nuclease-free water.
2.2 CRISPR/Cas9 Targeted Mutagenesis
-
1.
CRISPRscan (http://crisprscan.org): sgRNA candidate sequence design web portal (see Note 3).
-
2.
sgRNA target-specific oligonucleotide with the following structure:
-
5′-TAATACGACTCACTATA-[target-specific region, 20 bp]-GTTTTAGAGCTAGAA-3′ (Fig. 3b).
-
-
3.
Universal sgRNA reverse strand tail oligonucleotide: 5′-AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC-3′.
-
4.
sgRNA annealing mix solution: 3 μL 100 μM universal sgRNA reverse strand tail oligonucleotide, 4 μL nuclease-free water.
-
5.
Fluorometer.
-
6.
Fluorometer assay tubes.
-
7.
High-abundance RNA quantification kit (e.g., Qubit RNA BR, Invitrogen).
-
8.
1.25 μg/μL Cas9 protein with nuclear localization sequence stock solution.
-
9.
High-fidelity PCR kit.
-
10.
Forward and reverse primers designed to amplify genomic locus surrounding sgRNA target site.
-
11.
DNA extraction lysis buffer (e.g., QuickExtract™ DNA extraction solution lysis buffer, Lucigen).
-
12.
PCR cloning kit.
-
13.
Liquid bacterial cultures for plasmid amplification.
-
14.
DNA plasmid miniprep kit.
-
15.
pCS2-nCas9n plasmid for the expression of zebrafish codon optimized Cas9 mRNA (Addgene #47929).
-
16.
pBS-Nvec-codonopti-Cas9-2xNLS plasmid for the expression of a Nematostella vectensis codon-optimized Cas9 mRNA (Addgene #141108).
-
17.
mRNA synthesis kit.
2.3 Microinjection
- 1.
-
2.
Nematostella sperm water (see Note 7).
-
3.
5/6-Fluorescein isothiocyanate (FITC) stock solution: 20 mg/mL in Tris–HCl, pH > 9.5.
-
4.
Long-term labeling dextran conjugate (e.g., Dextran Texas Red™, 3000 molecular weight) stock solution: 20 mg/mL in nuclease-free water.
-
5.
Filamented thin wall glass capillary needles: 100 mm length, 1 mm diameter, 0.25 mm thickness (see Note 8).
-
6.
Needle puller (e.g., Sutter P-97).
-
7.
Micropipette capillary tips.
-
8.
Programmable microinjector.
-
9.
0.01 mm micrometer for needle calibration.
-
10.
60 mm petri dishes: BD Falcon – 351007 (see Note 9).
-
11.
12 ppt artificial sea water (ASW): 12 g/L artificial sea salt (e.g., Sea Salt, Instant Ocean™).
-
12.
Injection tracer dye solution: 1-part FITC stock solution: 1-part long-term labeling dextran conjugate solution (see Note 10).
-
13.
shRNA injection mixture: 0.5 μL injection tracer dye solution, x μL shRNA (to a final concentration of 500 ng/μL), nuclease-free water up to a total volume of 5 μL. Prepare fresh, keep on ice until ready for use.
-
14.
Ribonucleoprotein (RNP) injection mixture: 2 μL Cas9 protein with nuclear localization sequence, x μL sgRNA (to a final concentration of 500 ng/μL), nuclease-free water up to a total volume of 4.5 μL.
-
15.
Cas9 mRNA injection mixture: 0.5 μL injection tracer dye solution, x μL sgRNA (to a final concentration of 500 ng/μL), y μL Cas9 mRNA (see Table 1 for final suggested final concentrations), nuclease-free water up to a total volume of 5 μL.
2.4 Electroporation of shRNA
-
1.
Polysucrose 400 (e.g., Ficoll PM 400, Sigma Aldrich).
-
2.
Square wave electroporation system with electroporation cuvette chamber (e.g., ECM 830 Electro Square Porator, BTX; Gene Pulser Xcell ShockPod Cuvette Chamber, Bio-Rad).
-
3.
4 mm electroporation cuvettes.
-
4.
Electroporation Solution: 15% polysucrose 400 in 12 ppt ASW (see Note 11).
3 Methods
3.1 shRNA Production
-
1.
Identify the coding sequence (CDS) region for each gene of interest (see Note 12).
-
2.
Search the CDS for 19-mer shRNA candidate sequences using the shRNA prediction algorithm (see Notes 13 and 14).
-
3.
Select 3–5 candidate shRNA sequences for each gene, ensuring the selected sequences span different regions of target gene CDS.
-
4.
BLAST search each candidate in the Nematostella transcriptome using an expectation threshold of 1.0E-2 to compensate for shorter sequence inputs (see Note 15).
-
5.
To ensure the specificity of shRNAs, discard each candidate sequence that has more than one match in the transcriptome.
-
6.
Design gene-specific shRNA reverse oligos for 3–5 candidate shRNA sequences per gene using the following structure: 5′-AA-[shRNA candidate sequence, 19 bp]-TCTCTTGAA-[reverse complement of shRNA candidate sequence, 19 bp]-TATAGTGAGT-3′ (Fig. 3a, see Notes 16–18).
-
7.
Order the synthesized gene-specific shRNA oligos.
-
8.
Dilute universal shRNA primer and gene-specific reverse primer to 100 μM.
-
9.
Add 3 μL 100 μM gene-specific reverse primer to 7 μL shRNA annealing mix solution.
-
10.
Incubate annealing reaction at 70 °C for 2 min in a thermocycler.
-
11.
Hold annealing reaction at room temperature for 5 min.
-
12.
Add 10 μL of DNA template extension solution to 10 μL of annealing reaction.
-
13.
Incubate at 37 °C for 30 min.
-
14.
Incubate at 70 °C for 20 min to deactivate the DNA polymerase enzyme. This reaction mixture is the DNA Template and will be directly used for in vitro transcription.
-
15.
Use 5.5 μL DNA Template as starting product in a 20 μL in vitro transcription reaction per manufacturer’s instructions (see Note 19).
-
16.
Incubate in vitro transcription reaction at 37 °C for 5 h (see Note 20).
-
17.
Add 1 μL of RNase-free DNaseI to in vitro transcription reaction (see Note 21).
-
18.
Incubate at 37 °C for another 15 min to digest the DNA template.
-
19.
Purify shRNA using the RNA purification kit according to the manufacturer’s instructions (see Note 22).
-
20.
Add 80 μL nuclease-free water to expand the reaction mixture to 100 μL.
-
21.
Add 100 μL of ice cold 100% ethanol.
-
22.
Mix well by pipetting.
-
23.
Add the mixture into the RNA purification column.
-
24.
Centrifuge for 30 s at 10,000 rcf.
-
25.
Replace the flow-through with 700 μL of RNA wash buffer.
-
26.
Centrifuge for 30 s at 10,000 rcf.
-
27.
Discard flow-through.
-
28.
Centrifuge for 2 min at max speed to completely remove the RNA wash buffer.
-
29.
Transfer the column to a new microcentrifuge tube.
-
30.
Carefully add 35 μL of nuclease-free water to the membrane of the column.
-
31.
Wait for 1 min to allow the elution water to incubate on membrane at RT.
-
32.
Centrifuge for 1 min at maximum speed to elute RNA product.
-
33.
Quantify shRNA concentration using a spectrophotometer (see Note 23).
-
34.
shRNA can be stored at −20 °C for at least a month. For long-term storage, store at −80 °C for up to a year.
3.2 shRNA Microinjection
An alternative protocol for shRNA delivery using electroporation is provided in Subheading 3.3. When choosing which shRNA delivery method, it can be important to consider your experiment. The main benefit of microinjection is consistent shRNA delivery due to calibrated injection volumes and the use of long-term labeling injection dyes. Therefore, ideal experiments for shRNA microinjection may include functional screening of a single or a small number of genes of interest or optimization of a consistent shRNA phenotype for production of a consistent samples for further analysis.
-
1.
Load 2.2 μL shRNA injection mixture into a pulled capillary needle.
-
2.
Let the mixture fall to the tip of the needle (see Note 24).
-
3.
Insert the loaded needle into the capillary holder of the microinjector.
-
4.
Gently transfer 200–400 de-jellied eggs to the center of a 60-mm petri dish using a 1.5-mL transfer pipette. Eggs should adhere to the surface of the petri dish (see Note 25).
-
5.
Trim needle using forceps (see Note 26).
-
6.
Calibrate injection parameters to allow for an injection volume of ~60–80 pL (~1.5% volume of the egg) using a 0.01 mm micrometer. Injection parameters will differ for individual microinjector setups. A well-calibrated needle should repeatedly produce an injection volume 5 μm in diameter as measured using a micrometer (see Notes 27–29).
-
7.
Inject approximate number of eggs needed for experiment.
-
8.
Remove un-injected eggs from the dish with a 1.5-mL transfer pipette being careful to avoid injected eggs.
-
9.
Collect sperm water using a transfer pipette.
-
10.
Fertilize injected eggs by completely filling 60 mm dish.
-
11.
Repeat steps 1–10 to deliver non-targeting shRNA for the mock treatment group.
-
12.
Fertilize remaining de-jellied, untreated eggs to serve as a negative control.
-
13.
Incubate the fertilized embryos at room temperature (21–25 °C) overnight.
3.3 shRNA Electroporation
An alternative to this shRNA delivery method using microinjection is provided in Subheading 3.2.
When choosing which shRNA delivery method, it can be important to consider your experiment. The main benefits of electroporation are ease of shRNA delivery, rapid delivery to a large number of eggs, higher throughput genetic screening, low equipment cost, and potential applicability for use with other Cnidarian species where shRNA injection is very difficult or impossible [25]. Therefore, ideal experiments for shRNA electroporation may include genetic screening of a large number of genes as well as the opportunity for evolutionary comparison of gene function across different Cnidarian species.
-
1.
Transfer de-jellied Nematostella eggs into a 15-mL tube.
-
2.
Add 5 mL Nematostella sperm water.
-
3.
Fertilize for 30 min at room temperature by laying the tube horizontal and gently agitating every 5–10 min (see Note 30).
-
4.
Place the tube upright.
-
5.
Wait for the eggs to settle.
-
6.
Remove sperm water with transfer pipette.
-
7.
Wash the fertilized eggs 3 times with 10 mL of 12 ppt ASW.
-
8.
Transfer eggs in ~1 mL 12 ppt ASW to a clean microcentrifuge tube.
-
9.
Wait for eggs to settle.
-
10.
Resuspend eggs in electroporation solution to reach a dilution of 2–5 eggs/μL (see Notes 31 and 32).
-
11.
Gently pipette 100 μL of eggs suspension into a 4 mm electroporation cuvette using a p200 pipette with a disposable tip widened by cutting the end off.
-
12.
Add purified shRNA to a final concentration of 300 ng/μL directly to egg suspension in the cuvette.
-
13.
Mix gently by manually agitating the cuvette for approximately 15–20 s (see Note 33).
-
14.
Turn on electroporator.
-
15.
Insert cuvette into electroporation chamber.
-
16.
Set desired electroporation conditions (50 V, 25 ms, 1 pulse) (see Note 34).
-
17.
Press the start button to initiate electroporation.
-
18.
Confirm that the electroporation charge was successfully dispatched across the sample visually by checking for bubbles on the side of the cuvette near the meniscus of the electroporation solution.
-
19.
Remove the cuvette from chamber.
-
20.
Transfer eggs to a 60-mm petri dish using a transfer pipette.
-
21.
Use fresh 12 ppt ASW to rinse the cuvette of any remaining eggs into the 60-mm dish.
-
22.
Repeat step 21 until all eggs have been transferred into the 60-mm dish (see Note 35).
-
23.
Gently swirl dish to disperse electroporated eggs (see Note 36).
-
24.
Repeat steps 11–23 to deliver non-targeting shRNA for the mock treatment group.
-
25.
Fertilize remaining de-jellied, untreated eggs to serve as a negative control.
-
26.
Incubate the dishes at room temperature overnight.
3.4 Screening and Care of shRNA-Treated Animals
-
1.
Gently transfer developing blastula embryos into new 60 mm dishes filled with fresh 12 ppt ASW using a 1.5-mL transfer pipette.
-
2.
Discard unhealthy, unfertilized, or lysed animals as well as debris.
-
3.
Repeat steps 1 and 2 daily.
-
4.
As development proceeds, morphological, molecular or cellular phenotypes may begin to appear. Always compare experimental shRNA-treated groups to both mock treatments as well as to untreated wild type controls from the same spawning group (see Notes 37–41).
3.5 sgRNA Production for CRISPR/Cas9-Targeted Mutagenesis
-
1.
Identify the genomic region of interest (see Note 42).
-
2.
Copy and paste DNA sequence into the CRISPRscan Sequence Submission web interface (https://www.crisprscan.org/?page=sequence), select “Cas9 – NGG” and “In vitro T7 promoter” from dropdown menus and click “Get sgRNAs” button (see Notes 43–45).
-
3.
The program will suggest many different sgRNA sequences (in upper case) and will automatically design necessary oligo sequences for production by T7 in vitro transcription for each (Fig. 3b).
-
4.
Select 3–5 candidate sgRNA sequences for each DNA sequence (see Note 46).
-
5.
BLAST search each sgRNA sequence (upper case only from CRISPRscan-designed oligos) in the Nematostella genome using an expectation value of 1.0E-2 to compensate for shorter sequence inputs. Each candidate sequence should only have one match in the genome. Additionally, it is important to confirm that the sgRNAs avoid intron–exon junctions (see Notes 15 and 47).
-
6.
Order synthesized target-specific sgRNA oligos.
-
7.
Dilute universal sgRNA tail reverse and target-specific sgRNA oligos to 100 μM.
-
8.
Add 3 μL 100 μM target-specific sgRNA oligonucleotide to 7 μL sgRNA annealing mix solution.
-
9.
Complete sgRNA production by following steps 10–32 from Subheading 3.1 (Fig. 3b′).
-
10.
Quantify the concentration using fluorometer and high abundance RNA concentration kit (see Notes 48 and 49).
-
11.
Separate purified sgRNAs into 2–4 μL aliquots to avoid frequent freeze–thaw cycles. Aliquots can be stored at −20 °C for several weeks or moved to −80 °C for longer term storage if desired.
3.6 sgRNA/Cas9 Delivery by Microinjection
-
1.
Incubate RNP Injection Mixture at 37 °C for 10 min.
-
2.
Add 0.5 μL of injectable tracer dye solution.
-
3.
Mix by pipetting.
-
4.
Keep reaction at room temperature and protected from light until injection (see Note 50).
-
5.
Load 2.2 μL RNP injection mixture into a pulled capillary needle.
-
6.
Let the mixture fall to the tip of the needle.
-
7.
Insert the loaded needle into the capillary holder of the microinjector.
-
8.
Follow steps 4–12 from Subheading 3.2 to complete the injection of eggs.
-
9.
Incubate all experimental embryos at 18–27 °C overnight (see Note 51; Table 1).
-
10.
Follow steps 1–4 from Subheading 3.4 to observe potential phenotypes (see Notes 52 and 53).
3.7 Genotypic Screening and Care of CRISPR/Cas9 Injected Animals
Genotypic screening by standard PCR and molecular biology techniques is required to confirm DNA cutting and genomic mutagenesis.
-
1.
Collect 8–16 individual larvae (72 h post-fertilization or later) in PCR tubes, making sure to note those with an observable candidate phenotype. Be sure to include injection control and wild-type animals for downstream genotypic analysis.
-
2.
Carefully remove as much 12 ppt ASW as possible.
-
3.
Add 12 μL of DNA extraction lysis buffer (see Note 54).
-
4.
Lyse sample by incubating for 1 h at 65 °C.
-
5.
Incubate 3 min at 98 °C.
-
6.
Ensure tissue is fully lysed before placing tube on ice (see Note 55).
-
7.
Perform 50 μL PCR for each sample with high-fidelity PCR kit and gene-specific primers designed to amplify the region surrounding the target cut site.
-
8.
Run 2 μL of PCR product on a 1% agarose gel.
-
9.
Note reactions with a band of the correct size.
-
10.
Purify the remaining 48 μL of any positive PCR by column clean up.
-
11.
Submit PCR product to standard Sanger sequencing.
-
12.
Upon receipt of sequencing results, look for loss of base-calling quality and reduction of chromatogram peaks near target cut site. As these injected animals are F0, you can expect them to be mosaic and harbor several different alleles of genomic region of interest if mutagenesis was successful (see Note 56).
-
13.
Use Sanger sequencing information to correlate genotypic and phenotypic information if possible.
-
14.
Feed and raise the remaining F0 animals from injection series that show genotypic indications of successful mutagenesis (see Notes 57 and 58).
-
15.
If only a small number of CRISPR/Cas9-injected F0 animals show evidence of genomic cutting at this step, repeat Subheading 3.6 with increased concentration of sgRNA, Cas9 protein, or both in the RNP injection mixture (see Note 59; Table 1). If this increase is unsuccessful too, consider using Cas9 mRNA injection mixture in targeted mutagenesis experiments (see Notes 60–64; Table 1).
-
16.
Upon sexual maturation, isolate F0 animals in individual wells of a six-well untreated tissue culture plate.
-
17.
Spawn F0 animals in individual wells.
-
18.
Cross to either WT sperm or WT eggs to obtain heterozygous progeny. F1 animals resulting from this cross will not be individually mosaic, but some will be germline heterozygous carriers for the allele of interest (+/−).
-
19.
The population of F1 animals will likely exhibit many different mutant alleles as well as the wild-type allele. To ensure that a loss of function allele is identified, screen for and identify specific alleles for downstream functional characterization (see Note 65).
-
20.
Feed and raise F1 progeny from populations that showed genotypic evidence of desired mutant alleles (see Note 66).
-
21.
When F1 animals mature to the juvenile polyp stage (6–8 tentacle), separate individuals into wells of a six-well tissue culture plate.
-
22.
Cut 1–2 tentacles from each animal and transfer cut tissue to PCR tube for genotypic identification of specific mutant alleles. Be sure to maintain correlating information between tissue sample and animal (see Note 67).
-
23.
Lyse tissue in 15 μL lysis buffer by incubating at 65 °C for 3 h followed by 98 °C for 3 min (see Note 68).
-
24.
Perform a 50 μL PCR reaction on each sample with specific forward and reverse primers to amplify the target cut site and surrounding genomic region.
-
25.
Run 2 μL of each PCR product on a gel.
-
26.
Note reactions with a band of the correct size.
-
27.
Ligate PCR products of correct size into a cloning vector.
-
28.
Amplify 4–8 copies of the vector by liquid bacterial cultures.
-
29.
Perform miniprep of liquid cultures per manufacturer’s suggestions to isolate plasmid DNA containing genomic PCR insert of interest.
-
30.
Identify heterozygous carriers of mutant allele by Sanger sequencing (see Note 69).
-
31.
Continue raising F1 progeny with sequence-confirmed mutant alleles until sexual maturity (see Note 70).
-
32.
Identify F1 heterozygous animals of different sexes that carry the same mutant allele.
-
33.
If unable to identify F1 heterozygous animals of different sexes that carry the same allele, trans-heterozygous crosses of different mutant alleles of same gene can also be informative for gene function and phenotypic specificity (see Note 71).
-
34.
Spawn animals and cross to generate F2 animals.
-
35.
Separate individual F2 embryos and quantify morphological phenotypes. For a recessive mutant allele, approximately 25% of F2 animals should be homozygous mutant for the genomic region of interest (see Note 72).
-
36.
Perform genotypic analysis of phenotypically quantified F2 animals by repeating steps 4–13 to identify homozygous mutant animals and confirm mendelian segregation. Be sure to maintain individual information to correlate genotypic and phenotypic information (see Notes 73 and 74).
-
37.
Heterozygous F1 founders as well as heterozygous or homozygous F2 animals (and any generations beyond) can be maintained for future experiments or to share with other researchers (see Notes 75 and 76).
4 Notes
-
1.
The authors prefer the web-based tool siRNA Wizard™ from InvivoGen (https://www.invivogen.com/sirnawizard/design_advanced.php); however, any web-based or downloadable siRNA/shRNA design software should work.
-
2.
Commercially provided buffers that come with DNA polymerase (e.g., New England BioLabs 10× Buffer 2 or 10× Buffer 2.1) will also work.
-
3.
There are currently many websites and offline resources available for designing sgRNAs for use in the CRISPR/Cas9 system. We have had success with “CRISPRscan” and have structured this protocol on the design and output from this specific tool. Other websites should also work in this pipeline however it is important to confirm that the final target-specific sgRNA oligo structure detailed here is maintained.
-
4.
A sex-sorted bowl containing only confirmed sexually mature female Nematostella should be used as the source for Nematostella eggs.
-
5.
Induction of spawning and de-jellying of eggs have been previously reported in detail in previous protocols [15, 26] and should be consulted prior to the completion of any experimental protocols detailed here.
-
6.
Contamination of eggs during spawning or de-jellying process will result in induction of fertilization prior to completion of experimental manipulations which could have deleterious effects on experimental performance and outcome.
-
7.
It is not critical that the spawning bowl used for sperm water be only male Nematostella animals. If egg sacs are present in the spawned male bowl, avoid collecting those egg sacs when transferring sperm water for fertilization.
-
8.
Using a Sutter Model P-97 Horizontal Needle Puller, the following settings can serve as a good starting point for needle production. Heat = Ramp Test Value +15, Pull = 175, Velocity = 90, Time = 50, Pressure = 500.
-
9.
In general, eggs adhere best to polystyrene dishes. However, the authors suggest using BD Falcon—catalog number #351007 for 60 mm dishes specifically for any injection experiments as these dishes have provided consistent and robust egg adherence.
-
10.
FITC is a green, non-toxic, short-term tracer dye which allows direct visualization of the injection mix for up to 5 h inside experimental embryos. This enables control of the injection volume and allows for the easy elimination of un-injected eggs. Dextran Texas Red™ is a red, non-toxic, long-term tracer dye that will last for at least 7 days within injected animals. However, since it is much harder to visualize injection in the red channel of a fluorescent dissecting scope, it is generally recommended to add FITC to the mixture even when using Dextran as a long-term tracer. Alternatively, Dextran AlexaFluor488 (Thermo Fisher, D34682; Stock Concentration = 8.3 μg/μL) can be used as single long-term dye that works well for injection . However, it should be noted that primary polyps frequently exhibit endogenous green autofluorescence surrounding the pharynx which may make long-term labeling with this green dextran conjugates less ideal for many experiments.
-
11.
Polysucrose 400 can be difficult to dissolve for the Electroporation Solution. Heating can help or the solution can be left to nutate overnight. The Electroporation Solution should be clear and transparent [15].
-
12.
Double check the accuracy of the annotated gene model for your genomic locus or mRNA sequence by PCR or by cross-referencing with published RNA-seq results. shRNAs designed within intronic regions or against exon–intron junctions typically exhibit no knockdown effect.
-
13.
Most shRNA design tools will provide many candidate shRNA sequences. Select for shRNA sequences with the following features: (1) overall GC content between 40% and 55%; (2) low 3′ GC percentage; and (3) reasonable sequence complexity, as defined as a Shannon entropy value greater than or equal to 2 (many shRNA design algorithms will perform this part of the selection automatically, but if unsure, there are webtools (e.g., https://planetcalc.com/2476/) that can be helpful in quantifying this value).
-
14.
Sequences starting with GG or GGG usually have higher yields than sequences starting with GN. This is due to the nature of the T7 RNA polymerase. The composition of the initial sequence, however, does not seem to affect the effectiveness of shRNA .
-
15.
At the time of publication, the most readily accessible genomic resources for Nematostella vectensis through the Joint Genome Institute (https://mycocosm.jgi.doe.gov/Nemve1/Nemve1.home.html). Originally published in 2007 [27], this genome is a powerful resource for the identification of putative gene homologs and has served the field well for over a decade. However, users should also be mindful that this genome assembly is non-contiguous, containing reads from numerous individual animal genotypes and existing across almost 11,000 scaffolds and, therefore, potentially imperfect in some of its more formal annotations. As improved genomic resources for Nematostella vectensis emerge, this protocol can and should be used with the most up-to-date and trusted assemblies.
-
16.
We suggest designing 3–5 candidate sequences to try to increase the likelihood of finding 2 or more shRNAs that specifically and effectively knockdown the target gene of interest.
-
17.
A brief summary of each component of the gene-specific oligos and their functional importance. 5′-AA: after in vitro transcription, this generates a 3′-UU overhang, which mimics the terminator sequence of endogenous pre-microRNA. The 9 nt loop sequence, TCTCTTGAA, was first designed by Brummelkamp et al. (2002) and is crucial for shRNAs to be recognized and cleaved by the Dicer complex in vivo [28]. The 3′ sequence, TATAGTGAGT, reverse complements part of the T7 promoter sequence, enabling the Klenow reaction to generate a double-stranded DNA template for in vitro transcription.
-
18.
Gene-specific oligonucleotides should be exactly 59 bp long, which is below the length limit for standard oligonucleotide synthesis reactions of most companies, reducing costs.
-
19.
Make sure all components of the kit (with the exception of the T7 polymerase and RNase Inhibitor) are thawed to room temperature before use. The 10× transcription buffers can sometimes form a white precipitate at the bottom of the tube during this process. Vortex or invert the tube to completely dissolve the precipitant before adding the buffer to the reaction mix. Add all components according to the order listed in the manufacturer’s protocol . Due to the high salt concentration in the T7 reaction buffer, NTPs will precipitate if they are added to the buffer directly. Instead, add the reaction buffer last before adding the enzyme and quickly pipette to ensure homogenous distribution of the buffer without precipitating NTPs. If precipitation is observed in the transcription reaction mix, heat the mixture to 37 °C for 5–10 min then proceed with in vitro transcription reaction preparation and incubation.
-
20.
Incubation beyond 5 h does not significantly increase production yield for most commercially available kits. However, the reaction can be kept in the thermocycler at 4 °C overnight.
-
21.
By the end of the in vitro transcription reaction, white precipitate is frequently visible at the bottom of the tube. When adding the RNase-free DNaseI, make sure to pipette until the precipitate forms an even suspension.
-
22.
The kit suggests using any type of TRI reagent® (TRIzol®, RNAzol®, QIAzol®, etc.) for transcription reaction purification, which we found to be optional in the case of shRNA . In fact, incomplete removal of the TRI reagent can affect the quality of shRNA (an abnormally low 260/280 reading) and result in injection toxicity. If the user decides to use TRI reagent®, simply expand the reaction to 100 μL and add 300 μL of TRI reagent®. Mix well and add another 400 μL of 100% ethanol before proceeding according to the instruction from the kit. If no TRI reagent® is being used, the user can safely skip the first two washes with RNA prewash buffer as detailed in this protocol . Empirical testing in our laboratory of RNA purification of IVT reactions described here have shown no significant reduction in product yield when omitting the TRI reagent®; however, individual users may want to confirm this on their own.
-
23.
The concentration of shRNA by spectrophotometer after purification is usually 2000–3500 ng/μL, with a 260/280 ration above 1.9. A single transcription reaction typically yields a total of 60–100 μg of shRNA . This is sufficient for 50–100 rounds of injection or at least 1 round of electroporation, depending on the concentration needed.
-
24.
Bubbles in the injection mix can impair injection efficiency. If any bubbles are present in the mixture after loading, attempt to remove them by gently agitating the needle prior to mounting on microinjector. If bubbles cannot be eliminated, reload a new needle.
-
25.
For injection , gently eject de-jellied eggs from a transfer pipet into a 60-mm polystyrene dish filled with 12 ppt ASW and allow them to adhere on their own. Eggs will only adhere once, so be careful not to jostle them once plated.
-
26.
For initial needle trimming, trim needle tip just above to the “curvy” part that forms during pulling. A well-trimmed needle should show minimal wavering when moved.
-
27.
This calibration protocol is based on an injection volume of approximately 60–80 pL per egg, or ~1.5% total egg volume. Empirically, we have defined this as a 5 μm diameter bubble as measured by injecting into a drop of mineral oil on top of a 0.01 mm micrometer (diameter of 5 hashmarks).
-
28.
Microinjector setting will need to be determined on each machine and each injection session. Typical starting settings for an Eppendorf Femtojet are within the following ranges and could serve as a starting point for all microinjector models: injection pressure: 400–1200 hectopascals (hPa); injection duration: 0.1–0.7 s; compensation pressure: 5–30 hPa. In all instances, increasing a parameter increases injection volume and bubble size. “Injection Duration” seems to be the condition that can be most readily changed without damage to eggs.
-
29.
Additionally, to achieve and maintain the ~80 pL target volume while keeping the needle opening as narrow as possible, we regularly clean the needle using the microinjector “Clean” function, raising the needle out of the water, and by gently scraping the side of the needle using jeweler’s forceps. It is important to be careful not to trim or damage the needle once it is calibrated. If the needle is damaged or trimmed, recalibration will need to be performed.
-
30.
Electroporation of shRNA can also be performed on unfertilized eggs if desired. This can be particularly helpful if you are unsure of sperm quality/amount or wish to fertilize with multiple different male animals. If using unfertilized eggs, the protocol is the same except plenty of sperm water from the spawned males should be used for egg transfer and added to each dish following electroporation at step 21 in Subheading 3.3.
-
31.
The volume of egg suspension in electroporation solution should be determined by the number of electroporation experiments to be performed. In general, it is good to aim for at least 200 eggs in a volume of 100 μL for each electroporation experiment. For example, for three experiments, at least 500 μL of eggs in electroporation solution would be needed (including controls).
-
32.
The purpose of the polysucrose 400 supplement is to keep eggs in suspension for optimal electroporation. However, even with this supplement added, eggs can still settle to the bottom of the tube. If this happens, gently tap or rock the tube to resuspend the eggs.
-
33.
Electroporation requires substantially more shRNA than microinjection as the total delivery volume is ~100 μL (i.e., 300 ng/μL × 100 μL = 30 μg total). shRNA concentrations less than 2000 ng/μL are likely not sufficiently concentrated for electroporation. Consider repeating IVT with a smaller elution volume.
-
34.
These electroporation conditions have been experimentally validated for shRNA delivery into Nematostella eggs. Electroporation conditions for other stages of development in Nematostella have not been determined at time of this publication. Recently, parameters were reported for the electroporation of shRNA into the eggs of another Cnidarian, Hydractinia symbiolongicarpus [25], suggesting this could be a useful means of shRNA delivery in all Cnidarians.
-
35.
In addition to visual check in step 18 Subheading 3.3, some eggs should be morphologically abnormal (resembling the shmoo of a fission yeast) following transfer into a 60-mm petri dish. This is expected and a good sign that the eggs were successfully electroporated. Eggs should recover and return to normal morphology within 30–60 min.
-
36.
All Nematostella eggs can fuse during early embryogenesis, but electroporated eggs seem to be more prone to fusion than non-electroporated. For this reason, it is important to be sure to disperse the eggs throughout the dish prior to overnight incubation.
-
37.
Even under normal conditions, a certain percentage of Nematostella larvae and juveniles will look abnormal. It is recommended to keep a dish of non-injected or non-electroporated wild-type embryos as a negative biological control. Multiple shRNAs targeting the same gene should consistently result in similar phenotypes with high penetrance in comparison to the negative technical control of non-targeted shRNA injection . Additionally, delivery of shRNA can sometimes result in a slight delay of embryogenesis compared to untreated controls.
-
38.
Nematostella embryogenesis and development to the primary polyp stage takes approximately 7 days at room temperature (22 °C). Information regarding the expression pattern and expression level of the gene of interest (in situ hybridization, qPCR, or cross-referencing published RNA-seq results) can be extremely helpful when considering when and where to focus on potential phenotypes.
-
39.
If no phenotypes are readily apparent, we have observed that higher shRNA concentrations are more effective at knocking down genes expressed at later stages of development or at very high levels. At room temperature, gene knockdown by shRNA typically does not last longer than 7–10 days. If the gene of interest is expressed at the late stages of larval development or at primary polyp stage, shRNA injection may not have an effect, and it may be better to consider CRISPR/Cas9-targeted mutagenesis.
-
40.
Different genes may exhibit different sensitivity toward shRNA knockdown. Interestingly, shRNA does not cause obvious toxicity. Even when embryos were injected with 1500 ng/μL shRNA , >80% of injected embryos were viable. The exact working concentration for each shRNA may have to be determined empirically. We have observed gene-specific phenotypes at shRNA concentrations ranging from 100 to 1500 ng/μL.
-
41.
It can also be helpful to have primer sequences to generate positive and negative control shRNAs, their working concentrations, and observable phenotype [11, 15]:
-
shAnthox1a_R:
5′-AAGGTCTGACGACGAATGTGATCTCTTGAATCACATTCGTCGTCAGACCTATAGTGAGT-3′;
Working injection concentration: 500 ng/μL;
Working electroporation concentration: 900 ng/μL;
Observable phenotype: loss of endodermal segment s5, tentacle fusion at polyp stage.
-
shβ-catenin_R:
5′-AAGTGGCACCAAACGTATCATTCTCTTGAAATGATACGTTTGGTGCCACTATAGTGAGT-3′;
Working injection concentration: 100 ng/μL;
Working electroporation concentration: 300 ng/μL;
Observable phenotype: gastrulation failure, disruption of epithelial tissue and lethal at 4 dpf.
-
sheGFP_R:
5′-AAGACGTAAACGGCCACAAGTTCTCTTGAAACTTGTGGCCGTTTACGTCTATAGTGAGT-3′;
Working injection concentration: 100–1000 ng/μL;
Working electroporation concentration: 200 ng/μL;
Observable phenotype: reduction of GFP in transgenic animals. Knockdown effect lasts to 7 dpf when used at 1000 ng/μL.
-
Scrambled_R:
5′-AAGCAACACGCAGAGTCGTAATCTCTTGAATTACGACTCTGCGTGTTGCTATAGTGAGT-3′;
Working concentration: negative control, always match your test shRNA ;
Observable phenotype: no observable phenotype when used under 1500 ng/μL. Slight developmental delay when used at 2000 ng/μL.
-
-
42.
If possible, it can be a good idea to double check the accuracy of a genomic locus by PCR. Additionally, be aware of potential exon-intron boundaries as these are typically not ideal candidates to target for mutagenesis for functional analysis of gene function.
-
43.
The T7 promoter sequence (TAATACGACTCACTATA) at 5′ end is to allow for amplification of sgRNA by T7 RNA polymerase. Reverse complement of universal sgRNA tail oligo (GTTTTAGAGCTAGAA) is located 3′ to the target-specific sequence and allows for DNA template production suitable for IVT by Klenow reaction.
-
44.
Selecting “Sea anemone-Nematostella vectensis” from the dropdown menu will use JGI genome sequence information to detect potential off-target effects. However, if the exact sequence is not found in the JGI genome version (perhaps due to SNPs), CRISPRscan will display an error message.
-
45.
CRISPRscan is a predictive algorithm designed to predict in vivo cutting efficacy from previous data generated using >1000 sgRNAs injected in zebrafish [14]. sgRNAs are given scores to predict their ability to cut genomic DNA. Scores above 0.55 are termed “efficient for cutting” and above 0.70 are called “highly efficient for cutting (17).” In general, the direct correlation between CRISPRscan score and cutting efficiency holds true in Nematostella. However, it is worth noting that we have seen productive mutagenesis using sgRNAs with scores as low as 40 and when considering which sgRNA to use, the genomic location of the target with regard to ideal site for the experiment should be weighted more heavily than a CRISPRscan score. Both canonical (“GG18”) and non-canonical (“Gg18,” “GG17,” etc.) will work for cutting [14].
-
46.
If attempting to create frameshift mutations by non-homologous end-joining, choose 3–4 sgRNAs close to the start codon of the gene, within a known promoter region, or within the DNA sequence for key protein domains. sgRNAs targeting regions far from the transcription start site may be more likely to leave active or partially active gene products.
-
47.
“Sea anemone—Nematostella vectensis ” can be selected from the species dropdown menu on the CRISPRscan website genome; however, this source has no annotated information from the JGI genome and can only be used to show potential off target effects by sequence similarity, not to confirm functionality of a sgRNA target site.
-
48.
A spectrophotometer could also be used to quantify sgRNA concentration. However, in practice, we typically use a fluorometer and high-abundance RNA detection kit for sgRNA measurement as this method is more sensitive and sgRNAs as RNPs appear more toxic than shRNAs.
-
49.
Ideally, the original concentration should be above 2500 ng/μL. Additionally, if desired, sgRNA integrity can be check by electrophoresis. A clear ~100 bp band should appear for a good quality sgRNA.
-
50.
sgRNA/Cas9 protein mixtures can be reused at least two times if stored at −80 °C.
-
51.
Lower temperatures may be better for embryo survival, but the mutation rate of Cas9 protein increases with temperature. We have tried culturing embryos at temperatures as high as 29 °C and seen productive cutting. See Table 1 for additional information.
-
52.
It can be helpful to reference expression data for the targeted gene of interest when determining the best plan for observing phenotypes. shRNA knockdown data can also be helpful in this pursuit. However, as sgRNA/Cas9 animals will be mosaic, not all individuals harboring mutant alleles will show a phenotype as F0 animals.
-
53.
If injected embryos present with very low viability (10% survival) the day after injection , consider reducing the concentration of Cas9 protein in the RNP injection mixture. If injected embryos present with very high viability (>90% or similar to un-injected controls), consider increasing sgRNA concentrations as cutting may be low. A measurable reduction in viability can be a good indicator of efficient DNA cutting. See Table 1 for more information.
-
54.
The volume of lysis buffer can range from 5 to 15 μL depending on the developmental stage. In general, embryonic stages (blastula or gastrula) will require less lysis buffer (5–10 μL) than later planula or primary polyp stages (12–15 μL).
-
55.
Check tubes for any remaining tissue. If intact tissue is still present, pipette vigorously to help with lysis or, if that fails, incubate longer (up to overnight) at 65 °C.
-
56.
This step DOES NOT identify a specific allele or alleles present in the F0. Instead, this is a simple test to see if any evidence of genome cutting can be detected at the target site. Always compare sgRNA/Cas9-injected samples to wild-type or control-injected samples from the same experiment as SNPs or other natural genomic heterogeneity can exist in Nematostella. There are programs that can be used to try and predict the most prevalent mutant allele (i.e., TIDE, ICE, etc.), if desired. At this stage, the most important goal is to detect efficient DNA cutting. If allele-specific information is required at this stage, we would suggest cloning the PCR product into a cloning vector and sequencing many colonies (at least 12–16 colonies/PCR sample) from the resulting transformed bacteria. Ideal candidates for loss-of-function alleles are nonsense mutations that introduce a premature stop codon or large insertion or deletions that disrupt codon usage (e.g., insertions or deletions of a number of base pairs indivisible by 3). It should be noted though that presence or absence of a specific allele at this stage does not guarantee transmission to the F1 generation.
-
57.
It can be helpful to sort out the animals that present with a visible phenotype that is correlated with a genotype and see if they grow. Phenotypically abnormal animals are good candidates for transmission if the phenotype does not disrupt growth and sexual maturation.
-
58.
It can be helpful to use positive and negative control sgRNA examples when beginning CRISPR/Cas9-targeted mutagenesis. The following examples are from published work and adapted for the protocol described here as necessary [11, 29]. It should be noted that different genomic loci very regularly require individually optimized injection mixtures.
-
eGFP sgRNA (negative control for wild type animals):
5′-taatacgactcactataGGTCAGGGTGGTCACGAGGGgttttagagctagaa-3′;
Working sgRNA injection concentration: 250–500 ng/μL;
Working Cas9 protein injection concentration: 500 ng/μL;
Observable F0 phenotype: No observable phenotype in wild-type animals; reduction or loss of fluorescent protein expression in GFP transgenic lines [11].
-
eGFP genotyping oligo Forward: 5′-AAGGCGTTATGGTCGGTATG-3′
eGFP genotyping oligo Reverse: 5′-TGCTTGTCGGCCATGATATAG-3′,
-
APC (positive control for wild-type animals):
5′-taatacgactcactataGGGGGGCCCTAGTCAGCAGGgttttagagctagaa-3′;
Working sgRNA injection concentration: 500 ng/μL;
Working Cas9 protein concentration: 500 ng/μL;
Observable F0 phenotype: Formation of ectopic oral structures (tentacles and pharynx) at primary polyp stage, 7–10 dpf [27].
APC genotyping oligo Forward: 5′-AGAATCCTGCAGAAGATGAACA-3′
APC genotyping oligo Reverse: 5′-CCTGGCATACAAAGGTGACA-3’.
-
-
59.
In general, it is a good practice to attempt increasing sgRNA concentration before Cas9 protein to limit nonspecific toxicity as well as maintain an easy to work with RNP injection mixture. If increased sgRNA concentration alone does not increase genomic mutagenesis, increase both sgRNA and Cas9 protein. If injected embryo survival is significantly affected (0–20% viability), reduce Cas9 protein concentration until embryo viability returns to approximately 50% survival. The inherent differences in RNP cutting efficiency is why it is always best to start with 3–5 sgRNAs for the same target gene of interest.
-
60.
Cas9 recombinant protein is the first choice for targeted mutagenesis as high levels of nonspecific toxicity is observed with the injection of Cas9 mRNA. However, in instances where no phenotypes were observed with the injection of Cas9 protein, the injection of mRNA has resulted in targeted mutations. If you consistently see no DNA cutting across a range of sgRNA/Cas9 protein conditions, consider using Cas9 mRNA. Both zebrafish codon-optimized Cas9 mRNA and Nematostella codon-optimized Cas9 mRNA are functional in Nematostella embryos. Nematostella codon-optimized Cas9 mRNA exhibits mutagenesis rates similar to Cas9 protein and higher than the zebrafish codon-optimized version. However, again, it must be stressed that toxicity is often high with any Cas9 mRNA. See Table 1 for additional information.
-
61.
Nematostella vectensis codon-optimized Cas9 was designed by computationally determining the codon usage rate for each amino acid from the coding regions of all transcripts found in published Nematostella transcriptomes. Each codon in the Cas9 CDS was replaced with the most-frequently occurring codon for that amino acid, as determined above.
-
62.
Omit step 1 from Subheading 3.6 as no pre-injection incubation is necessary if using Cas9 mRNA.
-
63.
At step 4 in Subheading 3.6, store injection mixtures containing any Cas9 mRNA on ice until use.
-
64.
At step 9 in Subheading 3.6, incubate Cas9 mRNA injected embryos at 18 °C for higher viability.
-
65.
If desired, F1 embryos can undergo same genotypic analysis as done previously for F0 animals (steps 4–13 from Subheading 3.7). Base-calling may not be as reduced at target site as with F0 mosaic animals, but if a mutation has been transmitted sequencing errors should still be frequent after the target site. Again, this type of analysis is only to determine if any genomic editing has occurred. Specific alleles are not likely to be identified by this step.
-
66.
Make note and continue to care for any F0 animals that transmitted mutant alleles to their F1 progeny. Transmitting F0 animals can be useful to generate additional F1 animals for genotypic screening or for preliminary experiments.
-
67.
Other small amounts of adult tissue, such as part of the physa, can be used for genotyping if desired. However, it is important that the animal is still able to continue feeding and growing after tissue is taken.
-
68.
Adult tissue takes longer to lyse than tissue from earlier developmental stages. If tissue is not completely lysed following incubation, mix vigorously with a pipette and/or incubate sample longer.
-
69.
This is the step where specific mutant alleles will be identified. We suggest sequencing at least four colonies as this will give you an approximately 95% probability of identifying a mutant allele if present ((1 − (0.5)4) × 100% = 93.75%). Sequencing eight colonies gives a > 99% probability of identifying a potential mutant allele ((1 − (0.5)8) × 100% = 99.61%).
-
70.
It is a good practice to raise more than one mutant allele if possible. This will increase the likelihood of identifying a loss of function mutation. Additionally, testing multiple alleles that show the same phenotype confirms specificity.
-
71.
Trans-heterozygous crosses cross can be performed with F1 founder animals of different mutant alleles or even transmitting F0 animals. However, if crossing transmitting F0 animals, genotypic analysis must be performed following the cloning of the allelic PCR into a plasmid, as in steps 17–25 from Subheading 3.7, except using embryonic tissue instead of juvenile/adult tissue for DNA extraction.
-
72.
Based on F0 mutagenesis, shRNA knockdown or other experiments, a more detailed phenotypic analysis by immunohistochemistry or in situ hybridization analysis may be desired. We have successfully extracted DNA for PCR from samples post-antibody staining and post-in situ hybridization using the protocol described here in steps 4–13 from Subheading 3.7.
-
73.
A quantitative phenotypic analysis and corresponding genotypic confirmation of phenotype at F2 generation is the gold standard of all CRISPR/Cas9-targeted mutagenesis experiments. All resulting genotypes of F1 heterozygous cross can be readily identified from Sanger sequencing of a purified PCR product. Homozygous mutant animals will possess only one mutant allele, wild-type animals will possess only one non-mutant allele, and heterozygous animals will show loss of base-calling confidence at or after the target site indicating the presence of two alleles.
-
74.
Genotypic and phenotypic analysis will likely need to be performed for each experiment resulting from a F1 heterozygote cross.
-
75.
If homozygous mutants are viable and can become sexually mature, homozygous mutant F2 animals will be most helpful for future experiments as subsequent genotypic analysis is unnecessary.
-
76.
Animals not being actively used can be maintained at room temperature or 18 °C for years with weekly or biweekly feeding. Be sure to always clean 1–2 days after any feeding. For induction of spawning, move animals to 18 °C at least 1 week prior to target spawn date and feed heavily (every 1 or 2 days).
References
Technau U, Genikhovich G, Kraus JEM (2015) Cnidaria. In: Wanninger A (ed) Evolutionary developmental biology of invertebrates 1. Springer-Verlag, Wien, pp 115–163
Genikhovich G, Technau U (2017) On the evolution of bilaterality. Development 144:3392–3404
Technau U, Steele RE (2011) Evolutionary crossroads in developmental biology: Cnidaria. Development 138:1447–1458
Layden MJ, Rentzsch F, Röttinger E (2016) The rise of the starlet sea anemone Nematostella vectensis as a model system to investigate development and regeneration. Wiley Interdiscip Rev. Dev Biol 5:408–428
Warner JF, Guerlais V, Amiel AR et al (2018) NvERTx: a gene expression database to compare embryogenesis and regeneration in the sea anemone Nematostella vectensis. Development 145:dev162867
Amiel AR, Johnston HT, Nedoncelle K et al (2015) Characterization of Morphological and Cellular Events Underlying Oral Regeneration in the Sea Anemone. Nematostella vectensis. Int J Mol Sci 16(28449–28):471
Bossert PE, Dunn MP, Thomsen GH (2013) A staging system for the regeneration of a polyp from the aboral physa of the anthozoan Cnidarian Nematostella vectensis. Dev Dyn 242:1320–1331
Passamaneck YJ, Martindale MQ (2012) Cell proliferation is necessary for the regeneration of oral structures in the anthozoan cnidarian Nematostella vectensis. BMC Dev Biol 12:34
Bossert P, Thomsen GH (2017) Inducing Complete Polyp Regeneration from the Aboral Physa of the Starlet Sea Anemone Nematostella vectensis. J Vis Exp 119:e54626
Bely AE, Nyberg KG (2010) Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol 25:161–170
He S, del Viso F, Chen C-Y et al (2018) An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. Science 361:1377–1380
Moran Y, Fredman D, Praher D et al (2014) Cnidarian microRNAs frequently regulate targets by cleavage. Genome Res 24:651–663
Moran Y, Agron M, Praher D et al (2017) The evolutionary origin of plant and animal microRNAs. Nat Ecol Evol 1:0027
Karabulut A, He S, Chen C-Y et al (2019) Electroporation of short hairpin RNAs for rapid and efficient gene knockdown in the starlet sea anemone, Nematostella vectensis. Dev Biol 448:7–15
Ikmi A, McKinney SA, Delventhal KM et al (2014) TALEN and CRISPR/Cas9-mediated genome editing in the early-branching metazoan Nematostella vectensis. Nat Commun 5:5486
Moreno-Mateos MA, Vejnar CE, Beaudoin J-D et al (2015) CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat Methods 12:982–988
Vejnar CE, Moreno-Mateos MA, Cifuentes D et al (2016) Optimized CRISPR-Cas9 system for genome editing in zebrafish. Cold Spring Harb Protoc 2016(10). https://doi.org/10.1101/pdb.prot086850
Jao L-E, Wente SR, Chen W (2013) Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A 110(13904–13):909
Sunagar K, Columbus-Shenkar YY, Fridrich A et al (2018) Cell type-specific expression profiling unravels the development and evolution of stinging cells in sea anemone. BMC Biol 16:108
Xu T, Rubin GM (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117:1223–1237
Golic KG, Lindquist S (1989) The FLP recombinase of yeast catalyzes site-specific recombination in the drosophila genome. Cell 59:499–509
Dang DT, Perrimon N (1992) Use of a yeast site-specific recombinase to generate embryonic mosaics in Drosophila. Dev Genet 13:367–375
Lakso M, Sauer B, Mosinger B et al (1992) Targeted oncogene activation by site-specific recombination in transgenic mice. Proc Natl Acad Sci U S A 89:6232–6236
Orban PC, Chui D, Marth JD (1992) Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A 89:6861–6865
Quiroga-Artigas G, Duscher A, Lundquist K et al (2020) Gene knockdown via electroporation of short hairpin RNAs in embryos of the marine hydroid Hydractinia symbiolongicarpus. Sci Rep 10:12806
Genikhovich G, Technau U (2009) Induction of spawning in the starlet sea anemone Nematostella vectensis, in vitro fertilization of gametes, and dejellying of zygotes. Cold Spring Harb Protoc 2009(9). https://doi.org/10.1101/pdb.prot5281
Putnam NH, Srivastava M, Hellsten U et al (2007) Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317:86–94
Brummelkamp TR, Bernards R, Agami R (2002) A System for Stable Expression of Short Interfering RNAs in Mammalian Cells. Science 296:550–553
Kraus Y, Aman A, Technau U et al (2016) Pre-bilaterian origin of the blastoporal axial organizer. Nat Commun 7:11694
Acknowledgments
We thank all Gibson Lab members past and present for their help in the development and optimization of the protocols detailed here. We thank Mark Miller for illustrations. We also thank the Stowers Institute Molecular Biology Core, especially Kym Delventhal, MaryEllen Kirkman, and Kyle Weaver, for help with screening design as well as the Stowers Institute Reptile and Aquatics Core facility for animal husbandry help. This study was supported by NIH F32 GM131522 (EMH) and the Stowers Institute for Medical Research (MCG).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
Hill, E.M. et al. (2022). Manipulation of Gene Activity in the Regenerative Model Sea Anemone, Nematostella vectensis . In: Blanchoud, S., Galliot, B. (eds) Whole-Body Regeneration. Methods in Molecular Biology, vol 2450. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2172-1_23
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2172-1_23
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2171-4
Online ISBN: 978-1-0716-2172-1
eBook Packages: Springer Protocols






