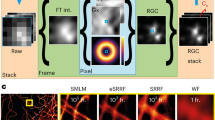
Abstract
Super-resolution Radial Fluctuations (SRRF) imaging is a computational approach to fixed and live-cell super-resolution microscopy that is highly accessible to life science researchers since it uses common microscopes and open-source software plugins for ImageJ. This allows users to generate super-resolution images using the same equipment, fluorophores, fluorescent proteins and methods they routinely employ for their studies without specialized sample preparations or reagents. Here, we discuss a step-by-step workflow for acquiring and analyzing images using the NanoJ-SRRF software developed by the Ricardo Henriques group, with a focus on imaging chromatin. Increased accessibility of affordable super-resolution imaging techniques is an important step in extending the reach of this revolution in cellular imaging to a greater number of laboratories.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dellaire G, Nisman R, Bazett-Jones DP (2004) Correlative light and electron spectroscopic imaging of chromatin in situ. Methods Enzymol 375:456–478
Guo J, Larabell CA (2019) Soft X-ray tomography: virtual sculptures from cell cultures. Curr Opin Struct Biol 58:324–332
Feng H, Wang X, Xu Z et al (2018) Super-resolution fluorescence microscopy for single cell imaging. Adv Exp Med Biol 1068:59–71
Jacquemet G, Carisey AF, Hamidi H et al (2020) The cell biologist’s guide to super-resolution microscopy. J Cell Sci 133
Gustafsson N, Culley S, Ashdown G et al (2016) Fast live-cell conventional fluorophore nanoscopy with ImageJ through super-resolution radial fluctuations. Nat Commun 7:12471
Laine RF, Tosheva KL, Gustafsson N et al (2019) NanoJ: a high-performance open-source super-resolution microscopy toolbox. J Phys D Appl Phys 52:163001
Acharya A, Bogdanov AM, Grigorenko BL et al (2017) Photoinduced chemistry in fluorescent proteins: curse or blessing? Chem Rev 117:758–795
Bagshaw CR, Cherny D (2006) Blinking fluorophores: what do they tell us about protein dynamics? Biochem Soc Trans 34:979–982
van de Linde S, Sauer M (2014) How to switch a fluorophore: from undesired blinking to controlled photoswitching. Chem Soc Rev 43:1076–1087
Dertinger T, Colyer R, Iyer G et al (2009) Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI). Proc Natl Acad Sci U S A 106:22287–22292
Culley S, Tosheva KL, Matos Pereira P et al (2018) SRRF: Universal live-cell super-resolution microscopy. Int J Biochem Cell Biol 101:74–79
Zhang X, Chen X, Zeng Z et al (2015) Development of a reversibly switchable fluorescent protein for super-resolution optical fluctuation imaging (SOFI). ACS Nano 9:2659–2667
Almada P, Culley S, Henriques R (2015) PALM and STORM: into large fields and high-throughput microscopy with sCMOS detectors. Methods 88:109–121
Stubb A, Laine RF, Miihkinen M et al (2020) Fluctuation-based super-resolution traction force microscopy. Nano Lett 20:2230–2245
Lee J, Salsman J, Foster J et al (2020) Lipid-associated PML structures assemble nuclear lipid droplets containing CCTα and Lipin1. Life Sci Alliance 3. https://doi.org/10.26508/lsa.202000751
Pinder J, Salsman J, Dellaire G (2015) Nuclear domain “knock-in” screen for the evaluation and identification of small molecule enhancers of CRISPR-based genome editing. Nucleic Acids Res 43:9379–9392
Bucevičius J, Keller-Findeisen J, Gilat T et al (2019) Rhodamine-Hoechst positional isomers for highly efficient staining of heterochromatin. Chem Sci 10:1962–1970
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Culley S, Albrecht D, Jacobs C et al (2018) Quantitative mapping and minimization of super-resolution optical imaging artifacts. Nat Methods 15:263–266
Lam AJ, St-Pierre F, Gong Y et al (2012) Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods 9:1005–1012
Shaner NC, Lambert GG, Chammas A et al (2013) A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nat Methods 10:407–409
Fabricius V, Lefèbre J, Geertsema H et al (2018) Rapid and efficient C-terminal labeling of nanobodies for DNA-PAINT. J Phys D Appl Phys 51:474005
Carrington G, Tomlinson D, Peckham M (2019) Exploiting nanobodies and Affimers for superresolution imaging in light microscopy. MBoC 30:2737–2740
de Beer MA, Giepmans BNG (2020) Nanobody-based probes for subcellular protein identification and visualization. Front Cell Neurosci 14:573278
Jež M, Bas T, Veber M et al (2013) The hazards of DAPI photoconversion: effects of dye, mounting media and fixative, and how to minimize the problem. Histochem Cell Biol 139:195–204
Nieuwenhuizen RPJ, Lidke KA, Bates M et al (2013) Measuring image resolution in optical nanoscopy. Nat Methods 10:557–562
Acknowledgments
Funding for microscope upgrades for SRRF imaging were funded by an Equipment Grant from the Dalhousie Medical Research Foundation (DMRF), and a Research, Tools, & Instruments (RTI) grant from the Natural Sciences and Engineering Research Council of Canada (NSERC). We would also like to thank Nvidia Corporation for the gift of the Titan V GPU used in this study obtained through their Higher Education and Research grants program. We would also like to thank Dr. Ricardo Henriques (Instituto Gulbenkian de Ciência, Portugal and University College London, UK) and his research group for their advice and access to the development-stage NanoJ-LiveSRRF software. Finally, we thank Dr. Gražvydas Lukinavičius and Dr. Jonas Bucevičius (Research group for chromatin labeling and imaging, Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) for the generous gift of the 5-TMR-Hoechst DNA dye used in this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Salsman, J., Dellaire, G. (2022). Super-Resolution Radial Fluctuations (SRRF) Microscopy. In: Heit, B. (eds) Fluorescent Microscopy. Methods in Molecular Biology, vol 2440. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2051-9_14
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2051-9_14
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2050-2
Online ISBN: 978-1-0716-2051-9
eBook Packages: Springer Protocols