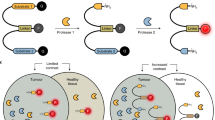
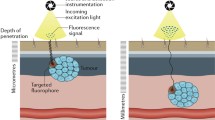
Abstract
Fluorescence (FL)-guided detection of cancer is one of the most promising approaches to achieve intraoperative assessment of surgical margins. Enzymes, such as aminopeptidase, carboxypeptidase, and glycosidase, whose activities are increased in cancer, have attracted great interest as imaging targets for rapid and sensitive visualization of cancerous tissues with fluorescent probes. Activatable probes, which are initially nonfluorescent but become strongly fluorescent upon rapid one-step cleavage of their substrate moiety by the target enzyme, are especially promising for practical clinical application during surgical or endoscopic procedures due to the highly amplified FL change generated by enzyme-catalyzed turnover at lesion sites. Here, we describe robust protocols for using activatable fluorescent probes targeting cancer-associated enzyme activities to visualize cultured cancer cells, metastatic cancer in a mouse model, and cancerous lesions in surgical specimens from patients.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Taxy JB (2009) Frozen section and the surgical pathologist: a point of view. Arch Pathol Lab Med 133(7):1135–1138. https://doi.org/10.1043/1543-2165-133.7.1135
Fukamachi K, Ishida T, Usami S, Takeda M, Watanabe M, Sasano H, Ohuchi N (2010) Total-circumference intraoperative frozen section analysis reduces margin-positive rate in breast-conservation surgery. Jpn J Clin Oncol 40(6):513–520. https://doi.org/10.1093/jjco/hyq006
Frangioni JV (2008) New technologies for human cancer imaging. J Clin Oncol 26(24):4012–4021. https://doi.org/10.1200/jco.2007.14.3065
Löning M, Diddens H, Küpker W, Diedrich K, Hüttmann G (2004) Laparoscopic fluorescence detection of ovarian carcinoma metastases using 5-aminolevulinic acid-induced protoporphyrin IX. Cancer 100(8):1650–1656. https://doi.org/10.1002/cncr.20155
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012:940585. https://doi.org/10.1155/2012/940585
Verdoes M, Oresic Bender K, Segal E, van der Linden WA, Syed S, Withana NP, Sanman LE, Bogyo M (2013) Improved quenched fluorescent probe for imaging of cysteine cathepsin activity. J Am Chem Soc 135(39):14726–14730. https://doi.org/10.1021/ja4056068
Tanei T, Pradipta AR, Morimoto K, Fujii M, Arata M, Ito A, Yoshida M, Saigitbatalova E, Kurbangalieva A, Ikeda J-I, Morii E, Noguchi S, Tanaka K (2018) Cascade reaction in human live tissue allows clinically applicable diagnosis of breast cancer morphology. Adv Sci (Weinh) 6(2):1801479. https://doi.org/10.1002/advs.201801479
Zhang C, Mao Y, Wang K, Tian J (2018) The identification of breast cancer by Near-Infrared fluorescence imaging with methylene blue. J Clin Oncol 36(15_Suppl):e12591. https://doi.org/10.1200/JCO.2018.36.15_suppl.e12591
Mahmood U, Weissleder R (2003) Near-infrared optical imaging of proteases in cancer. Mol Cancer Ther 2(5):489–496
Urano Y, Sakabe M, Kosaka N, Ogawa M, Mitsunaga M, Asanuma D, Kamiya M, Young MR, Nagano T, Choyke PL, Kobayashi H (2011) Rapid cancer detection by topically spraying a γ-glutamyl transpeptidase-activated fluorescent probe. Sci Transl Med 3(110):110ra119. https://doi.org/10.1126/scitranslmed.3002823
Ueo H, Shinden Y, Tobo T, Gamachi A, Udo M, Komatsu H, Nambara S, Saito T, Ueda M, Hirata H, Sakimura S, Takano Y, Uchi R, Kurashige J, Akiyoshi S, Iguchi T, Eguchi H, Sugimachi K, Kubota Y, Kai Y, Shibuta K, Kijima Y, Yoshinaka H, Natsugoe S, Mori M, Maehara Y, Sakabe M, Kamiya M, Kakareka JW, Pohida TJ, Choyke PL, Kobayashi H, Ueo H, Urano Y, Mimori K (2015) Rapid intraoperative visualization of breast lesions with γ-glutamyl hydroxymethyl rhodamine green. Sci Rep 5(1):12080. https://doi.org/10.1038/srep12080
Miampamba M, Liu J, Harootunian A, Gale AJ, Baird S, Chen SL, Nguyen QT, Tsien RY, González JE (2017) Sensitive in vivo visualization of breast cancer using ratiometric protease-activatable fluorescent imaging agent, AVB-620. Theranostics 7(13):3369–3386. https://doi.org/10.7150/thno.20678
Kawatani M, Yamamoto K, Yamada D, Kamiya M, Miyakawa J, Miyama Y, Kojima R, Morikawa T, Kume H, Urano Y (2019) Fluorescence detection of prostate cancer by an activatable fluorescence probe for PSMA carboxypeptidase activity. J Am Chem Soc 141(26):10409–10416. https://doi.org/10.1021/jacs.9b04412
Asanuma D, Sakabe M, Kamiya M, Yamamoto K, Hiratake J, Ogawa M, Kosaka N, Choyke PL, Nagano T, Kobayashi H, Urano Y (2015) Sensitive β-galactosidase-targeting fluorescence probe for visualizing small peritoneal metastatic tumours in vivo. Nat Commun 6(1):6463. https://doi.org/10.1038/ncomms7463
Sakabe M, Asanuma D, Kamiya M, Iwatate RJ, Hanaoka K, Terai T, Nagano T, Urano Y (2013) Rational design of highly sensitive fluorescence probes for protease and glycosidase based on precisely controlled spirocyclization. J Am Chem Soc 135(1):409–414. https://doi.org/10.1021/ja309688m
Ogasawara A, Kamiya M, Sakamoto K, Kuriki Y, Fujita K, Komatsu T, Ueno T, Hanaoka K, Onoyama H, Abe H, Tsuji Y, Fujishiro M, Koike K, Fukayama M, Seto Y, Urano Y (2019) Red fluorescence probe targeted to dipeptidyl peptidase-IV for highly sensitive detection of esophageal cancer. Bioconjug Chem 30(4):1055–1060. https://doi.org/10.1021/acs.bioconjchem.9b00198
Onoyama H, Kamiya M, Kuriki Y, Komatsu T, Abe H, Tsuji Y, Yagi K, Yamagata Y, Aikou S, Nishida M, Mori K, Yamashita H, Fujishiro M, Nomura S, Shimizu N, Fukayama M, Koike K, Urano Y, Seto Y (2016) Rapid and sensitive detection of early esophageal squamous cell carcinoma with fluorescence probe targeting dipeptidyl peptidase IV. Sci Rep 6(1):26399. https://doi.org/10.1038/srep26399
Komatsu T, Hanaoka K, Adibekian A, Yoshioka K, Terai T, Ueno T, Kawaguchi M, Cravatt BF, Nagano T (2013) Diced electrophoresis gel assay for screening enzymes with specified activities. J Am Chem Soc 135(16):6002–6005. https://doi.org/10.1021/ja401792d
Acknowledgments
This work was supported in part by AMED under grant number JP19gm0710008 (to Y.U.), by JST/PRESTO grant JPMJPR14F8 (to M.K.), by MEXT/JSPS KAKENHI grants JP16H02606, JP26111012, and JP19H05632 (to Y.U.) and JP15H05951 “Resonance Bio”, JP19H02826, and JP19K22242 (to M.K.), by JSPS Core-to-Core Program (grant number: JPJSCCA20170007), A. Advanced Research Networks, by Japan Foundation for Applied Enzymology (to M.K.), and The Naito Foundation (to M.K.), as well as a stipend from the Graduate Program for Leaders in Life Innovation (GPLLI) (to K.F.), World-leading Innovative Graduate Study Program for Life Science and Technology (WINGS-LST) (to K.F.), Masason Foundation (to K.F.) and a JSPS stipend (to K.F.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Fujita, K., Kamiya, M., Urano, Y. (2021). Rapid and Sensitive Detection of Cancer Cells with Activatable Fluorescent Probes for Enzyme Activity. In: Kim, SB. (eds) Live Cell Imaging. Methods in Molecular Biology, vol 2274. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-1258-3_17
Download citation
DOI: https://doi.org/10.1007/978-1-0716-1258-3_17
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-1257-6
Online ISBN: 978-1-0716-1258-3
eBook Packages: Springer Protocols