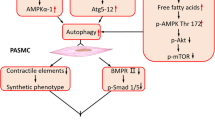
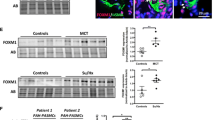
Abstract
Apoptosis (or programmed cell death) is a mechanism of cellular destruction that is essential for a variety of physiological events, such as tissue sculpturing during development and the removal of abnormal or damaged cells. However, untimely activation of this process can contribute to the pathogenesis and progression of a variety of human diseases, and pulmonary arterial hypertension is no exception. Recent evidence indicates that endothelial cell (EC) apoptosis is critically involved in the pathophysiological changes in PAH and that increased or decreased levels of apoptosis may contribute to different stages of the disease. The purpose of this chapter is to provide an overview of this rapidly evolving field and try to reconcile some of the apparent conflicts regarding the role of EC apoptosis in the initiation and progression of pulmonary hypertension. Considerable attention has been paid to the role of reduced apoptosis in other vascular cell types, particularly vascular smooth muscle cells (SMCs), which contributes to the massive medial growth and arterial remodeling characteristic of established disease, and this is reviewed in detail in other chapters. In contrast, this chapter will highlight the current concepts and controversies surrounding the role of abnormal EC growth and survival in the pathogenesis of PAH. In particular, the role of endothelial apoptosis as an initiating event in the development of PAH will be reviewed and current concepts pertaining to the emergence of hyperproliferative and apoptosis-resistant ECs in later stages of this disease will be discussed. This chapter also attempts to provide a unifying framework reconciling the apparent discrepancies between early apoptosis and EC cell loss which may represent one of the first events in the initiation of PAH, and the angioproliferative lesions that contribute characteristic pathological features of advanced disease.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Flemming W (1885) Über die Bildung von Richtungsfiguren in Saugethiereiern beim Untergang Graff’scher Follikel. Archive Anatomische Entwicklung Gesch (noe Anatomy and embryology) 221–224
Glücksmann A (1930) Ueber die Bedeutung von Zellvorgangen fur die Formbildung epithelialer Organe. 2. ges. Anat. I. 2. Anat Entw Gesch 93:35
Glücksmann A (1940) Development and differentiation of the tadpole eye. Br J Ophthal 24:153
Glucksmann A (1947) Cell counts in serial biopsies of carcinomata. In Recent Advances in Clinical Pathology, ed. Dyke, S. C. pp. 338–349. Churchill, London
Glücksmann AT, Tansley K (1936) Some effects of gamma radiation on the developing rat retina. Br J Ophthal 20:497
Spear FGG, Glücksmann A (1937) The effect of gamma radiation on cells in vivo. Br J Radiol 11:53
Williams CM (1961) The juvenile hormone. II. Its role in the endocrine control of molting, pupation, and adult development in the Cecropia silkworm. Biol Bull Woods Hole 121:572–585
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
O’Rourke MG, Ellem KA (2000) John Kerr and apoptosis. Med J Aust 173:616–617
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Vannini N, Pfeffer U, Lorusso G, Noonan DM, Albini A (2008) Endothelial cell aging and apoptosis in prevention and disease: E-selectin expression and modulation as a model. Curr Pharm Des 14:221–225
Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9:231–241
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Ashkenazi A, Dixit VM (1998) Death receptors: signaling and modulation. Science 281:1305–1308
Li P, Nijhawan D, Budihardjo I et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Sperandio S, de Belle I, Bredesen DE (2000) An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci U S A 97:14376–14381
Fishman AP (2004) A century of pulmonary hemodynamics. Am J Respir Crit Care Med 170:109–113
Wheeler EC, Brenner ZR (1995) Peripheral vascular anatomy, physiology, and pathophysiology. AACN Clin Issues 6:505–514
Winn RK, Harlan JM (2005) The role of endothelial cell apoptosis in inflammatory and immune diseases. J Thromb Haemost 3:1815–1824
Tuder RM, Cool CD, Geraci MW et al (1999) Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 159:1925–1932
Christman BW, McPherson CD, Newman JH et al (1992) An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 327:70–75
Geraci MW, Gao B, Shepherd DC et al (1999) Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J Clin Invest 103:1509–1515
Hoshikawa Y, Voelkel NF, Gesell TL et al (2001) Prostacyclin receptor-dependent modulation of pulmonary vascular remodeling. Am J Respir Crit Care Med 164:314–318
Badesch DB, Orton EC, Zapp LM et al (1989) Decreased arterial wall prostaglandin production in neonatal calves with severe chronic pulmonary hypertension. Am J Respir Cell Mol Biol 1:489–498
Aguilar RV, Farber HW (2000) Epoprostenol (prostacyclin) therapy in HIV-associated pulmonary hypertension. Am J Respir Crit Care Med 162:1846–1850
Barst RJ, McGoon M, McLaughlin V et al (2003) Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol 41:2119–2125
Barst RJ, Rubin LJ, Long WA et al (1996) A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 334:296–302
Rosenzweig EB, Kerstein D, Barst RJ (1999) Long-term prostacyclin for pulmonary hypertension with associated congenital heart defects. Circulation 99:1858–1865
Szczeklik J, Dubiel JS, Mysik M, Pyzik Z, Krol R, Horzela T (1978) Effects of prostaglandin E1 on pulmonary circulation in patients with pulmonary hypertension. Br Heart J 40:1397–1401
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376
Fagan KA, Fouty BW, Tyler RC et al (1999) The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J Clin Invest 103:291–299
Adnot S, Raffestin B, Eddahibi S (1995) NO in the lung. Respir Physiol 101:109–120
Giaid A, Saleh D (1995) Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 333:214–221
Mason NA, Springall DR, Burke M et al (1998) High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol 185:313–318
Balfour-Lynn IM, Laverty A, Dinwiddie R (1996) Reduced upper airway nitric oxide in cystic fibrosis. Arch Dis Child 75:319–322
Kaneko FT, Arroliga AC, Dweik RA et al (1998) Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 158:917–923
Channick RN, Newhart JW, Johnson FW et al (1996) Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: an ambulatory delivery system and initial clinical tests. Chest 109:1545–1549
Hoeper MM, Schwarze M, Ehlerding S et al (2000) Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. N Engl J Med 342:1866–1870
Yanagisawa M, Kurihara H, Kimura S et al (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411–415
Vanhoutte PM, Tang EH (2008) Endothelium-dependent contractions: when a good guy turns bad! J Physiol 586:5295–5304
Humbert M, Montani D, Perros F, Dorfmuller P, Adnot S, Eddahibi S (2008) Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vascul Pharmacol 49:113–118
Bohm F, Pernow J (2007) The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res 76:8–18
Coe Y, Haleen SJ, Welch KM, Coceani F (2000) The endothelin-A-receptor antagonist PD 180988 (CI-1034) selectively reverses the pulmonary vasoconstrictor response to hypoxia in the lamb. J Cardiovasc Pharmacol 36:S331–S333
Dupuis J, Prie S (1999) The ETA-receptor antagonist LU 135252 prevents the progression of established pulmonary hypertension induced by monocrotaline in rats. J Cardiovasc Pharmacol Ther 4:33–39
Kim H, Yung GL, Marsh JJ et al (2000) Endothelin mediates pulmonary vascular remodelling in a canine model of chronic embolic pulmonary hypertension. Eur Respir J 15:640–648
Stewart DJ, Levy RD, Cernacek P, Langleben D (1991) Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 114:464–469
Giaid A, Yanagisawa M, Langleben D et al (1993) Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328:1732–1739
Sitbon O, McLaughlin VV, Badesch DB et al (2005) Survival in patients with class III idiopathic pulmonary arterial hypertension treated with first line oral bosentan compared with an historical cohort of patients started on intravenous epoprostenol. Thorax 60:1025–1030
Channick R, Badesch DB, Tapson VF et al (2001) Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a placebo-controlled study. J Heart Lung Transplant 20:262–263
Givertz MM, Colucci WS, LeJemtel TH et al (2000) Acute endothelin A receptor blockade causes selective pulmonary vasodilation in patients with chronic heart failure. Circulation 101:2922–2927
Barst RJ, Langleben D, Badesch D et al (2006) Treatment of pulmonary arterial hypertension with the selective endothelin-A receptor antagonist sitaxsentan. J Am Coll Cardiol 47:2049–2056
Galie N, Badesch D, Oudiz R et al (2005) Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol 46:529–535
Loyd JE, Primm RK, Newman JH (1984) Familial primary pulmonary hypertension: clinical patterns. Am Rev Respir Dis 129:194–197
Nichols WC, Koller DL, Slovis B et al (1997) Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31-32. Nat Genet 15:277–280
Deng Z, Haghighi F, Helleby L et al (2000) Fine mapping of PPH1, a gene for familial primary pulmonary hypertension, to a 3-cM region on chromosome 2q33. Am J Respir Crit Care Med 161:1055–1059
Lane KB, Machado RD, Pauciulo MW et al (2000) Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Endothelium-dependent contractions: when a good guy turns bad. The International PPH Consortium. Nat Genet 26:81–84
Morrell NW (2006) Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc 3:680–686
Davies RJ, Morrell NW (2008) Molecular mechanisms of pulmonary arterial hypertension: role of mutations in the bone morphogenetic protein type II receptor. Chest 134:1271–1277
Deng Z, Morse JH, Slager SL et al (2000) Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 67:737–744
Rudarakanchana N, Flanagan JA, Chen H et al (2002) Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 11:1517–1525
Gaussin V, Van de Putte T, Mishina Y et al (2002) Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A 99:2878–2883
Kaneko H, Arakawa T, Mano H et al (2000) Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 27:479–486
Abe E, Yamamoto M, Taguchi Y et al (2000) Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J Bone Miner Res 15:663–673
Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22:233–241
Atkinson C, Stewart S, Upton PD et al (2002) Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105:1672–1678
Miyazono K, Maeda S, Imamura T (2005) BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev 16:251–263
Yamamura Y, Hua X, Bergelson S, Lodish HF (2000) Critical role of Smads and AP-1 complex in transforming growth factor-β-dependent apoptosis. J Biol Chem 275:36295–36302
Beppu H, Kawabata M, Hamamoto T et al (2000) BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 221:249–258
Zhang S, Fantozzi I, Tigno DD et al (2003) Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285:L740–L754
Morrell NW, Yang X, Upton PD et al (2001) Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-β1 and bone morphogenetic proteins. Circulation 104:790–795
Yang X, Long L, Southwood M et al (2005) Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res 96:1053–1063
Yang J, Davies RJ, Southwood M et al (2008) Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res 102:1212–1221
Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA et al (2006) Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res 98:209–217
Valdimarsdottir G, Goumans MJ, Rosendahl A et al (2002) Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation 106:2263–2270
Southwood M, Jeffery TK, Yang X et al (2008) Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J Pathol 214:85–95
Taraseviciene-Stewart L, Kasahara Y, Alger L et al (2001) Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15:427–438
Kido M, Du L, Sullivan CC, Deutsch R, Jamieson SW, Thistlethwaite PA (2005) Gene transfer of a TIE2 receptor antagonist prevents pulmonary hypertension in rodents. J Thorac Cardiovasc Surg 129:268–276
Austin ED, Loyd JE (2007) Genetics and mediators in pulmonary arterial hypertension. Clin Chest Med 28:43–57
Beppu H, Ichinose F, Kawai N et al (2004) BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 287:L1241–L1247
Long L, MacLean MR, Jeffery TK et al (2006) Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 98:818–827
Song Y, Jones JE, Beppu H, Keaney JF Jr, Loscalzo J, Zhang YY (2005) Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 112:553–562
Cool CD, Stewart JS, Werahera P et al (1999) Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol 155:411–419
Jamison BM, Michel RP (1995) Different distribution of plexiform lesions in primary and secondary pulmonary hypertension. Hum Pathol 26:987–993
Yamaki S, Wagenvoort CA (1981) Plexogenic pulmonary arteriopathy: significance of medial thickness with respect to advanced pulmonary vascular lesions. Am J Pathol 105:70–75
Masri FA, Xu W, Comhair SA et al (2007) Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293:L548–L554
Tuder RM, Flook BE, Voelkel NF (1995) Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest 95:1798–1807
Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S (1998) Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am J Respir Cell Mol Biol 18:768–776
Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF (2001) The pathobiology of pulmonary hypertension. Endothelium Clin Chest Med 22:405–418
Geiger R, Berger RM, Hess J, Bogers AJ, Sharma HS, Mooi WJ (2000) Enhanced expression of vascular endothelial growth factor in pulmonary plexogenic arteriopathy due to congenital heart disease. J Pathol 191:202–207
Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM (2001) Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 88:E2–E11
Campbell AI, Zhao Y, Sandhu R, Stewart DJ (2001) Cell-based gene transfer of vascular endothelial growth factor attenuates monocrotaline-induced pulmonary hypertension. Circulation 104:2242–2248
Partovian C, Adnot S, Raffestin B et al (2000) Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol 23:762–771
Zhao YD, Campbell AI, Robb M, Ng D, Stewart DJ (2003) Protective role of angiopoietin-1 in experimental pulmonary hypertension. Circ Res 92:984–991
Kugathasan L, Dutly AE, Zhao YD et al (2005) Role of angiopoietin-1 in experimental and human pulmonary arterial hypertension. Chest 128:633S–642S
Kugathasan L, Ray JB, Deng Y, Rezaei E, Dumont DJ, Stewart DJ (2009) The angiopietin-1-Tie2 pathway prevents rather than promotes pulmonary arterial hypertension in transgenic mice. J Exp Med 206:2221–2234
Dewachter L, Adnot S, Fadel E et al (2006) Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am J Respir Crit Care Med 174:1025–1033
Du L, Sullivan CC, Chu D et al (2003) Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med 348:500–509
Sullivan CC, Du L, Chu D et al (2003) Induction of pulmonary hypertension by an angiopoietin 1/TIE2/serotonin pathway. Proc Natl Acad Sci U S A 100:12331–12336
Golpon HA, Fadok VA, Taraseviciene-Stewart L et al (2004) Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 18:1716–1718
Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF (2005) Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19:1178–1180
Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF (2005) Vascular endothelial growth factor receptor blockade by SU5416 combined with pulsatile shear stress causes apoptosis and subsequent proliferation of apoptosis-resistant endothelial cells. Chest 128:610S–611S
Ingram DA, Caplice NM, Yoder MC (2005) Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood 106:1525–1531
Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC (2005) Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood 105:2783–2786
Hur J, Yoon CH, Kim HS et al (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24:288–293
Ingram DA, Mead LE, Tanaka H et al (2004) Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 104:2752–2760
Schechner JS, Nath AK, Zheng L et al (2000) In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A 97:9191–9196
Yoder MC, Mead LE, Prater D et al (2007) Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood 109:1801–1809
King J, Hamil T, Creighton J et al (2004) Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res 67:139–151
Stump MM, Jordan GL Jr, Debakey ME, Halpert B (1963) Endothelium grown from circulating blood on isolated intravascular dacron hub. Am J Pathol 43:361–367
Asahara T, Murohara T, Sullivan A et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Warburton D, Perin L, Defilippo R, Bellusci S, Shi W, Driscoll B (2008) Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thorac Soc 5:703–706
Kovacic JC, Moore J, Herbert A, Ma D, Boehm M, Graham RM (2008) Endothelial progenitor cells, angioblasts, and angiogenesis - old terms reconsidered from a current perspective. Trends Cardiovasc Med 18:45–51
Gehling UM, Ergun S, Schumacher U et al (2000) In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood 95:3106–3112
Peichev M, Naiyer AJ, Pereira D et al (2000) Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood 95:952–958
Prater DN, Case J, Ingram DA, Yoder MC (2007) Working hypothesis to redefine endothelial progenitor cells. Leukemia 21:1141–1149
Hristov M, Weber C (2004) Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med 8:498–508
Gulati R, Jevremovic D, Peterson TE et al (2003) Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 93:1023–1025
Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ (2005) Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 96:442–450
Michelakis ED, Webster L, Mackey JR (2008) Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer 99:989–994
McMurtry MS, Bonnet S, Wu X et al (2004) Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res 95:830–840
Barst RJ (2005) PDGF signaling in pulmonary arterial hypertension. J Clin Invest 115:2691–2694
Kerkela R, Grazette L, Yacobi R et al (2006) Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 12:908–916
Klein M, Schermuly RT, Ellinghaus P et al (2008) Combined tyrosine and serine/threonine kinase inhibition by sorafenib prevents progression of experimental pulmonary hypertension and myocardial remodeling. Circulation 118:2081–2090
Moreno-Vinasco L, Gomberg-Maitland M, Maitland ML et al (2008) Genomic assessment of a multikinase inhibitor, sorafenib, in a rodent model of pulmonary hypertension. Physiol Genomics 33:278–291
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Moudgil, R., Lalu, M.M., Stewart, D.J. (2011). Endothelial Apoptosis and Repair in Pulmonary Arterial Hypertension. In: Yuan, JJ., Garcia, J., West, J., Hales, C., Rich, S., Archer, S. (eds) Textbook of Pulmonary Vascular Disease. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-87429-6_28
Download citation
DOI: https://doi.org/10.1007/978-0-387-87429-6_28
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-0-387-87428-9
Online ISBN: 978-0-387-87429-6
eBook Packages: MedicineMedicine (R0)