Summary
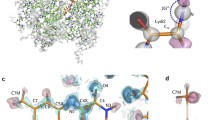
A high resolution structure is described of the lumen-side domain of cytochrome f. This structure is the first for a polypeptide subunit of cytochrome or bc1 or b6f complexes, and has at least three unique or unprecedented features for a c-type cytochrome: (i) more than one structural domain; (ii) predominantly β-strand; and (iii) the N-terminal α-amino group is the orthogonal (6th) ligand, which is unprecedented for a heme protein.
2,3 Å at the time of the writing of the article, now 1.96 Å.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Barker PD and Mauk AG (1992) pH-linked conformational regulation of a metalloprotein oxidation-reduction equilibrium. J Am Chem Soc 114: 3619–3624
Bergström J and Vänngard T (1982) EPR signals and orientation of cytochromes in the spinach chloroplast thylakoid membrane. Biochim Biophys Acta 682: 452–456
Crowder MS, Prince RC and Bearden A (1982) Orientation of membrane-bound cytochromes in chloroplasts, detected by low-temperature EPR spectroscopy. FEBS Lett 144: 204–208
Davenport HE and Hill R (1952) The preparation and some properties of cytochrome f. Proc Roy Soc London Ser B 139: 327–345
Davis DJ, Frame MK and Johnson DA (1988) Resonance Raman spectroscopy indicates a lysine as the sixth iron ligand in cytochrome f. Biochim Biophys Acta 936: 61–66
Doolittle RF (1994) Convergent evolution: The need to be explicit. Trends Biochem Sci 19: 15–18
Gray JC (1978) Purification and properties of cytochrome f from charlock. Eur J Biochem 82: 133–141
Gray JC (1992) Cytochrome f: Structure, function, and biosynthesis. Photosyn Res 34: 359–374
Gross EL (1993) Plastocyanin: Structure and function. Photosyn Res 37:103–116
Ho KK and Krogmann DW (1980) Cytochrome f from spinach and cyanobacteria. J Biol Chem 255: 3855–3861
Johnson EM, Schabelrauch LS and Sears BB (1991) A plastome mutation affects processing of both chloroplast and nuclear DNA-encoded plastid proteins. Mol Gen Genet 225: 106–112
Kirwin PM, Elderfield PD, Williams RS and Robinson C (1991) Transport of proteins into chloroplasts: Organization, orientation, and lateral distribution of the plastocyanin processing peptidase. J Biol Chem 263: 18128–18132
Kraulis PJ (1991) MOLSCRIPT: A program to produce detailed and schematic maps of protein structures. J Appl Crystallog 24: 946–950
Martinez SE, Smith JL, Huang D, Szczepaniak A and Cramer WA (1992) Crystallographic studies of the lumen-side domain of turnip cytochrome f. In: Murata N (ed) Research in Photosynthesis, Vol II, pp 495–498. Kluwer, Dordrecht
Martinez SE, Huang D, Szczepaniak A, Cramer WA and Smith JL (1994) Crystal structure of chloroplast cytochrome f reveals a novel cytochrome fold and unexpected heme ligation. Structure 2: 95–105
Moore GR and Pettigrew GW (1990) Cytochromes c: Evolutionary, Structural, and Physicochemical Aspects. Springer-Verlag, Berlin
Morand LZ, Frame MK, Colvert KK, Johnson DA, Krogmann DW and Davis DJ (1989) Plastocyanin-cytochrome f interaction. Biochemistry 28: 8039–8047
Pfanner N and Neupert W (1990) The mitochondrial protein import apparatus. Ann Rev Biochem 59: 331–353
Qin L and Kostic NM (1992) Electron transfer reactions of cytochrome f with flavin semiquinones and with plastocyanin. Importance of protein-protein interactions and of donor-acceptor coupling. Biochemistry 31: 5145–5150
Qin L and Kostic NM (1993) Importance of protein rearrangement in the electron-transfer reaction between the physiological partners cytochrome f and plastocyanin. Biochemistry 32: 6073–6080
Redinbo MR, Yeates TO and Merchant S (1994) Plastocyanin: Structural and functional analysis. J Bioenerg Biomemb 26: 49–66
Riedel A, Rutherford W, Hauska G, Müller A and Nitschke W (1991) Chloroplast Rieske center: EPR study on its spectral characteristics, relaxation, and orientation properties. J Biol Chem 266: 17838–17844
Rigby SEJ, Moore GR, Gray JC, Godsby PMA, George SJ and Thomson AJ (1988) NMR, EPR, and magnetic CD studies of cytochrome f. Biochem J 256: 571–577
Siedow JN, Vickery LE and Palmer G (1980) The nature of the axial ligands of cytochrome f. Arch Biochem Biophys 203: 101–107
Simpkin D, Palmer G, Devlin FJ, McKenna MC, Jensen GM and Stephens PJ (1989) The axial ligands of heme in cytochromes: A near infrared magnetic circular dichroism study of cytochromes c, c1, b, and spinach cytochrome f. Biochemistry 28: 8033–8039
Tae G-S, Furbacher PN and Cramer WA (1996) Evolution of cytochrome bc1 complexes. In: Baltscheffsky H (ed) Origin and Evolution of Biological Energy Conversion. VCH
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1996 Kluwer Academic Publishers
About this chapter
Cite this chapter
Martinez, S.E., Huang, D., Smith, J.L., Cramer, W.A. (1996). Some Consequences of the High Resolution X-Ray Structure Analysis of Cytochrome f. In: Ort, D.R., Yocum, C.F., Heichel, I.F. (eds) Oxygenic Photosynthesis: The Light Reactions. Advances in Photosynthesis and Respiration, vol 4. Springer, Dordrecht. https://doi.org/10.1007/0-306-48127-8_22
Download citation
DOI: https://doi.org/10.1007/0-306-48127-8_22
Publisher Name: Springer, Dordrecht
Print ISBN: 978-0-7923-3683-9
Online ISBN: 978-0-306-48127-7
eBook Packages: Springer Book Archive