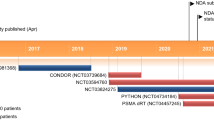
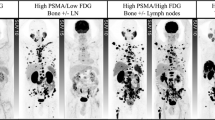
Abstract
Flotufolastat F 18 (POSLUMA®) is an 18F-labelled radiohybrid (rh) prostate-specific membrane antigen (PSMA)-targeted imaging agent being developed by Blue Earth Diagnostics, a subsidiary of Bracco Imaging, for prostate cancer imaging. In May 2023, flotufolastat F 18 received its first approval in the USA as a radioactive diagnostic agent for positron emission tomography (PET) of PSMA positive lesions in men with prostate cancer with suspected metastasis who are candidates for initial definitive therapy or with suspected recurrence based on elevated serum prostate-specific antigen (PSA) level. This article summarizes the milestones in the development of flotufolastat F 18 leading to this first approval.
Similar content being viewed by others
References
Murthy V, Aggarwal R, Koo PJ. The emerging role of next-generation imaging in prostate cancer. Curr Oncol Rep. 2022;24(1):33–42.
Werner RA, Derlin T, Lapa C, et al. 18F-labeled, PSMA-targeted radiotracers: leveraging the advantages of radiofluorination for prostate cancer molecular imaging. Theranostics. 2020;10(1):1–16.
Blue Earth Diagnostics. POSLUMA (flotufolastat F 18) injection, for intravenous use. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/216023s000lbl.pdf. Accessed 26 Jun 2023.
Blue Earth Diagnostics. U.S. FDA approves Blue Earth Diagnostics’ POSLUMA® (flotufolastat F 18) injection, first radiohybrid PSMA-targeted PET imaging agent for prostate cancer [media release]. 30 May 2023. https://www.businesswire.com/news/home/20230530005180/en/U.S.-FDA-Approves-Blue-Earth-Diagnostics%E2%80%99-POSLUMA%C2%AE-Flotufolastat-F-18-Injection-First-Radiohybrid-PSMA-targeted-PET-Imaging-Agent-for-Prostate-Cancer.
Bracco Imaging. Bracco Imaging launches new subsidiary, Blue Earth Therapeutics, to advance development of next generation therapeutic radiopharmaceutical technology [media release]. 1 Mar 2022. http://www.braccoimaging.com.
Blue Earth Diagnostics. Blue Earth Diagnostics and PETNET solutions announce new commercial supply agreement for axumin® (Fluciclovine F 18) and investigational 18F-rhPSMA-7.3 prostate cancer imaging agent [media release]. 16 Jun 2020. http://www.blueearthdiagnostics.com.
Blue Earth Diagnostics., Bracco Imaging. Bracco Imaging announces completion of acquisition of Blue Earth Diagnostics [media release]. 1 Aug 2019. http://www.blueearthdiagnostics.com.
Blue Earth Diagnostics. Blue Earth Diagnostics expands oncology portfolio with exclusive, worldwide licensing of investigational radiohybrid PSMA-targeted agents for prostate cancer from Scintomics [media release]. 2 May 2018. http://www.blueearthdiagnostics.com.
Blue Earth Diagnostics. Blue Earth Diagnostics acquires exclusive, worldwide rights to therapeutic applications of Scintomics radiohybrid prostate-specific membrane antigen (rhPSMA) technology for prostate cancer [media release]. 5 Jan 2021. http://www.blueearthdiagnostics.com.
Wurzer A, Parzinger M, Konrad M, et al. Preclinical comparison of four [18F, natGa]rhPSMA-7 isomers: influence of the stereoconfiguration on pharmacokinetics. EJNMMI Res. 2020;10(1):149.
Malaspina S, Oikonen V, Kuisma A, et al. Kinetic analysis and optimisation of 18F-rhPSMA-7.3 PET imaging of prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48(11):3723–31.
Langbein T, Wurzer A, Gafita A, et al. The influence of specific activity on the biodistribution of 18F-rhPSMA-7.3: a retrospective analysis of clinical PET data. J Nucl Med. 2022;63(5):742–5.
Malaspina S, Taimen P, Kallajoki M, et al. Uptake of 18F-rhPSMA-7.3 in positron emission tomography imaging of prostate cancer: a phase 1 proof-of-concept study. Cancer Biother Radiopharm. 2022;37(3):205–13.
Notohamiprodjo S, Eiber M, Lohrmann C, et al. Temporary reactive response of axillary lymph nodes to COVID-19 vaccination on 18F-rhPSMA-7.3 PET/CT in patients with prostate cancer. J Nucl Med. 2022;63(11):1673–6.
Tolvanen T, Kalliokoski K, Malaspina S, et al. Safety, biodistribution, and radiation dosimetry of 18F-rhPSMA-7.3 in healthy adult volunteers. J Nucl Med. 2021;62(5):679–84.
Chapin BF. Diagnostic performance and safety of 18F-rhPSMA-7.3 PET in patients with newly diagnosed prostate cancer: results from a phase 3, prospective multicenter study (LIGHTHOUSE) [abstract no. 134]. In: The 23rd Annual Meeting of the Socieity of Urologic Oncology 2022.
Koontz BF. Detection of true positive M1 lesions by 18F-rhPSMA-73 PET in newly diagnosed prostate cancer: results from the phase 3 prospective LIGHTHOUSE study [abstract no. 315]. J Clin Oncol. 2023;41(6 Suppl):315.
Blue Earth Diagnostics. Blue Earth Diagnostics announces additional results from phase 3 LIGHTHOUSE trial of investigational PET imaging agent 18F-rhPSMA-7.3 in newly diagnosed prostate cancer [media release]. 16 Feb 2023. http://www.blueearthdx.com.
Jani AB, Ravizzini GC, Gartrell BA, et al. Diagnostic performance and safety of 18F-rhPSMA-7.3 positron emission tomography in men with suspected prostate cancer recurrence: results from a phase 3, prospective, multicenter study (SPOTLIGHT). J Urol. 2023;210(2):299–311.
Langbein T, Wang H, Rauscher I, et al. Utility of 18F-rhPSMA-7.3 PET for imaging of primary prostate cancer and preoperative efficacy in N-staging of unfavorable intermediate- to very high-risk patients validated by histopathology. J Nucl Med. 2022;63(9):1334–42.
Kroenke M, Schweiger L, Horn T, et al. Validation of 18F-rhPSMA-7 and 18F-rhPSMA-7.3 PET imaging results with histopathology from salvage surgery in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2022;63(12):1809–14.
Rauscher I, Karimzadeh A, Schiller K, et al. Detection efficacy of 18F-rhPSMA-7.3 PET/CT and impact on patient management in patients with biochemical recurrence of prostate cancer after radical prostatectomy and prior to potential salvage treatment. J Nucl Med. 2021;62(12):1719–26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Young-A Heo is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Heo, YA. Flotufolastat F 18: Diagnostic First Approval. Mol Diagn Ther 27, 631–636 (2023). https://doi.org/10.1007/s40291-023-00665-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-023-00665-y