Abstract
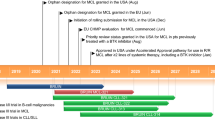
Zanubrutinib (Brukinsa®), an orally-administered Bruton tyrosine kinase (BTK) inhibitor, is being developed by BeiGene for the treatment of B-cell malignancies. Zanubrutinib received accelerated approval in the USA on 14 November 2019 for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy, based on overall response rate (ORR) seen in phase II and I/II clinical trials. This article summarizes the milestones in the development of zanubrutinib leading to this first approval for the treatment of MCL.
Similar content being viewed by others
References
Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–9.
Rickert RC. New insights into pre-BCR and BCR signalling with relevance to B cell malignancies. Nat Rev Immunol. 2013;13(8):578–91.
BeiGene. Brukinsa™ (zanubrutinib) capsules, for oral use: US Prescribing Information; 2019. https://www.brukinsa.com. Accessed 10 Dec 2019.
Dimopoulos M, Opat S, Lee HP, et al. Major responses in Myd88 wildtype (Myd88wt) Waldenstrom macroglobulinemia (WM) patients treated with Bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111) [abstract no. PF487]. HemaSphere. 2019;3:196.
BeiGene. BeiGene announces plan to pursue accelerated approval in the U.S. of BTK inhibitor zanubrutinib in Waldenstrm macroglobulinemia (WM) [media release]; 22 Jul 2018. http://www.beigene.com.
BieGene. BieGene Ltd. Form 10-K (28 February 2019); 2019. http://ir.beigene.com. Accessed 10 Dec 2019.
Catalent. Catalent to supply BeiGene’s BTK inhibitor BRUKINSA™ (zanubrutinib) [media release]. 21 Nov 2019. http://www.catalent.com.
Li CJ, Jiang C, Liu Y, et al. Pleiotropic action of novel Bruton’s tyrosine kinase inhibitor BGB-3111 in mantle cell lymphoma. Mol Cancer Ther. 2019;18(2):267–77.
Flinsenberg TWH, Tromedjo CC, Hu N, et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK cell effector function in patients with mantle cell lymphoma. Haematologica. 2019. https://doi.org/10.3324/haematol.2019.220590.
Tarantelli C, Zhang L, Curti E, et al. The Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) demonstrated synergies with other anti-lymphoma targeted agents. Haematologica. 2019;104(7):e307–9.
Hu N, Zhang S, He M, et al. BTK inhibitor BGB-3111 synergizes with lenalidomide in MCL models. Cancer Res. 2016;76:3.
Zou YX, Zhu HY, Li XT, et al. The impacts of zanubrutinib on immune cells in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hematol Oncol. 2019;37(4):392–400.
Zhu J, Li J, Zhou J, et al. BGB-3111, a highly specific BTK inhibitor, is well tolerated and highly active in Chinese patients with relapsed/refractory B-cell malignancies: initial report of a phase 1 trial in China [abstract]. Blood. 2017;130(Suppl 1).
Song Y, Zhou K, Zou D, et al. Zanubrutinib in patients with relapsed/refractory mantle cell lymphoma [abstract no. 015]. Hematol Oncol. 2019;37(Suppl 2):45–6.
Tam CS, Wang M, Simpson D, et al. Updated safety and efficacy data in the phase 1 trial of patients with mantle cell lymphoma (MCL) treated with bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111) [abstract no. 191]. Hematol Oncol. 2019;37(Suppl 2):245–7.
Trotman J, Opat S, Marlton P, et al. Updated safety and efficacy data in a phase 1/2 trial of patients with Waldenstrom macroglobulinaemia (WM) treated with the Bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111) [abstract no. PF481]. HemaSphere. 2019;3:192–3.
Trotman J, Tam CS, Marlton P, et al. Improved depth of response with increased follow-up for patients (PTS) with Waldenstrom macroglobulinemia (WM) treated with Bruton’s tyrosine kinase (BTK) inhibitor zanubrutinib [abstract no. PS1186]. HemaSphere. 2018;2(Suppl 2):537–8.
Trotman J, Opat S, Marlton P, et al. Bruton’s tyrosine kinase (BTK) inhibitor BGB-3111 demonstrates high very good partial response (VGPR) rate in patients with Waldenstrom macroglobulinemia (WM) [abstract no. 59]. Hematol Oncol. 2017;35(Suppl 2):70–1.
Tam CS, Robak P, Ghia P, et al. Efficacy and safety of zanubrutinib in patients with treatment-naive chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with Del(17p): initial results from arm C of the SEQUOIA (BGB-3111-304) trial [abstract no. 499]. In: American Society of Hematology annual meeting and exposition; 2019.
Xu W, Yang S, Zhou K, et al. Zanubrutinib for patients with relapsed or refractory chronic lymphocytic leukemia [abstract no. 049]. Hematol Oncol. 2019;37(Suppl 2):87–8.
Cull G, Simpson D, Opat S, et al. Treatment with the Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) demonstrates high overall response rate and durable responses in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): updated results from a phase 1/2 trial [abstract no. 500]. In: American Society of Hematology annual meeting and exposition; 2019.
Tam CS, Simpson D, Opat S, et al. Safety and activity of the highly specific BTK inhibitor BGB-3111 in patients with indolent and aggressive non-Hodgkin’s lymphoma [abstract]. Blood. 2017;130(Suppl 1).
Cull G, Opat S, Trotman J, et al. Safety and activity of the highly specific BTK inhibitor BGB-3111 in combination with the PD-1 inhibitor BGB-A317 in patients with B-cell lymphoid malignancies [abstract]. Blood. 2017;130(Suppl 1).
Tam CS, Quach H, Nicol A, et al. Zanubrutinib plus obinutuzumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) or relapsed/refractory (R/R) follicular lymphoma (FL) [abstract no. 075]. Hematol Oncol. 2019;37(Suppl 2):121–2.
Tam CS, Opat S, Zhu J, et al. Pooled analysis of safety data from monotherapy studies of the Bruton tyrosine kinase (BTK) inhibitor, zanubrutinib (BGB-3111), in B-cell malignancies [abstract no. PS1159]. HemaSphere. 2019;3:526.
BeiGene. BeiGene announces results of phase 3 ASPEN trial of zanubrutinib compared to ibrutinib for the treatment of patients with Waldenström’s macroglobulinemia [media release]. 2019. http://ir.beigene.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yahiya Syed is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.11344898.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Syed, Y.Y. Zanubrutinib: First Approval. Drugs 80, 91–97 (2020). https://doi.org/10.1007/s40265-019-01252-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01252-4