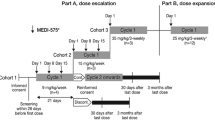
Abstract
Olaratumab (Lartruvo™) is a fully human IgG1 monoclonal antibody targeted against the human platelet-derived growth factor (PDGF) receptor α (PDGFRα). It was developed by Eli Lilly and Co. (previously ImClone Systems) after PDGFRα was identified as a potential therapeutic target in a variety of cancers. Olaratumab acts by selectively binding PDGFRα, thereby blocking PDGF ligand binding and inhibiting PDGFRα activation and downstream signalling. In October 2016, olaratumab received its first global approval, in the USA, for use in combination with doxorubicin for the treatment of adult patients with soft tissue sarcoma. The approval was granted by the US FDA under its Accelerated Approval Program based on the results of the JGDG phase II trial (NCT01185964). In addition, the EMA granted conditional approval for olaratumab in this indication in November 2016 following a review under the EMA’s Accelerated Assessment Program. An international, confirmatory phase III trial in patients with soft tissue sarcoma is ongoing (ANNOUNCE; NCT02451943). Olaratumab has also been investigated in phase II trials in several other cancers. This article summarizes the milestones in the development of olaratumab leading to this first approval, for use in combination with doxorubicin for the treatment of soft tissue sarcoma in adults.
Similar content being viewed by others
References
Loizos N, Xu Y, Huber J, et al. Targeting the platelet-derived growth factor receptor α with a neutralizing human monoclonal antibody inhibits the growth of tumor xenografts: implications as a potential therapeutic target. Mol Cancer Ther. 2005;4(3):369–79.
US FDA. Lartruvo (olaratumab) injection: US prescribing information. 2016. http://www.fda.gov. Accessed 27 Oct 2016.
European Medicines Agency. Lartruvo (olaratumab): summary of product characteristics. 2016. http://www.ema.europa.eu. Accessed 02 Dec 2016.
Shah GD, Loizos N, Youssoufian H, et al. Rationale for the development of IMC-3G3, a fully human immunoglobulin G subclass 1 monoclonal antibody targeting the platelet-derived growth factor receptor α. Cancer. 2010;116(4 Suppl):1018–26.
Heldin C-H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal. 2013;11:97.
Pietras K, Rubin K, Sjöblom T, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62(19):5476–84.
Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet. 2016;388(10043):488–97.
World Health Organization Classification of Tumours. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®): soft tissue sarcoma (Version 2.2016). 2016. http://www.nccn.org. Accessed 02 Dec 2016.
Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415–23.
Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: a phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;34(32):3898–905.
European Medicines Agency. New treatment for patients with soft tissue sarcoma: Lartruvo recommended for conditional approval [media release]. 2016. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2016/09/WC500212892.pdf. Accessed 02 Dec 2016.
Eli Lilly and Co. FDA grants Priority Review for Lilly’s olaratumab, an investigational medicine for advanced soft tissue sarcoma [media release]. 2016. http://lilly.mediaroom.com/index.php?s=9042&item=137539. Accessed 02 Dec 2016.
US FDA. Orphan drug designations and approvals: olaratumab. 2014. https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=448414. Accessed 02 Dec 2016.
European Medicines Agency. Public summary of opinion on orphan designation: olaratumab for the treatment of soft tissue sarcoma. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Orphan_designation/2015/04/WC500185238.pdf. Accessed 02 Dec 2016.
Updates. Summary of recent deal activity: drug discovery technologies. Int J Pharm Med. 2005;19(5-6):349–59.
Eli Lilly and Co. Lilly completes acquisition of ImClone Systems [media release]. 2008. http://lilly.mediaroom.com/index.php?s=9042&item=118078. Accessed 02 Dec 2016.
Bristol-Myers Squibb. Bristol-Myers Squibb completes acquisition of Medarex, Inc. [media release]. 2009. http://news.bms.com/press-release/financial-news/bristol-myers-squibb-completes-acquisition-medarex-inc. Accessed 02 Dec 2016.
Gerber DE, Gupta P, Dellinger MT, et al. Stromal platelet-derived growth factor receptor α (PDGFRα) provides a therapeutic target independent of tumor cell PDGFRα expression in lung cancer xenografts. Mol Cancer Ther. 2012;11(11):2473–82.
Matei D, Emerson RE, Lai Y-C, et al. Autocrine activation of PDGFRα promotes the progression of ovarian cancer. Oncogene. 2006;25(14):2060–9.
Stock P, Monga D, Tan X, et al. Platelet-derived growth factor receptor-α: a novel therapeutic target in human hepatocellular cancer. Mol Cancer Ther. 2007;6(7):1932–41.
Russell MR, Liu Q, Fatatis A. Targeting the α receptor for platelet-derived growth factor as a primary or combination therapy in a preclinical model of prostate cancer skeletal metastasis. Clin Cancer Res. 2010;16(20):5002–10.
Villalobos V, Agulnik M, Pollack SM, et al. A phase I open-label study to evaluate the effect of olaratumab on the pharmacokinetics (PK) of doxorubicin (Dox) in patients with advanced soft tissue sarcoma (STS) [abstract no. CT145]. Cancer Res. 2016;76(14 Suppl).
Gerber DE, Campbell TC, Swanson P, et al. A randomized phase 2 study of a human antiplatelet-derived growth factor α (PDGFRα) monoclonal antibody (olaratumab, IMC-3G3) with paclitaxel/carboplatin or paclitaxel/carboplatin alone in previously untreated patients with advanced non-small cell lung cancer (NSCLC) [abstract no. 8050]. In: 2014 ASCO annual meeting. 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the author on the basis of scientific completeness and accuracy. M. Shirley is a salaried employee of Adis, Springer SBM.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Shirley, M. Olaratumab: First Global Approval. Drugs 77, 107–112 (2017). https://doi.org/10.1007/s40265-016-0680-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0680-2