Abstract
Background
Particular interest has been generated regarding the possible influence of statin use on sleep quality. However, no conclusive evidence exists that a particular statin is more likely to be associated with sleep disturbances versus others. It remains uncertain whether different statins produce different risks for sleep disturbance.
Objective
To examine the association between statin use and the risk of sleep disturbances, we conducted data mining using the US Food and Drug Administration Adverse Event Reporting System (FAERS) and a large organized database of prescriptions constructed by a database vendor (Japan Medical Information Research Institute, Inc. Japan).
Methods
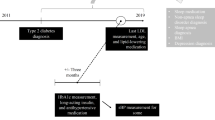
Relevant reports in the FAERS were identified and analyzed. Data from the first quarter of 2004 through the end of 2013 were included in this study. The reporting odds ratio (ROR) was used to detect spontaneous report signals, calculated using the case/non-case method. For the ROR, a signal was detected if the lower limit of 95 % two-sided confidence interval (95 % CI) was >1. Additionally, signal detection using the IC was conducted using the IC025 metric, a lower limit of the 95 % CI of the IC, where a signal is detected if the IC025 value exceeds 0. In addition, symmetry analysis was used to identify the risk of insomnia after using statins over the period of January 2006 to August 2013.
Results
In the analyses of the FAERS database, significant signals for sleep disturbances including disturbances in initiating and maintaining sleep, sleep disorders NEC, sleeping disorders due to a general medical condition, and parasomnias were found. In the prescription sequence symmetry analysis, a significant association between statin use and hypnotic drug use was found, with adjusted sequence ratios of 1.14 (1.03–1.26), 1.20 (1.11–1.29), and 1.18 (1.11–1.25) at intervals of 91, 182, and 365 days, respectively.
Conclusion
Multi-methodological approaches using different algorithms and databases strongly suggest that statin use is associated with an increased risk for sleep disturbances including insomnia.
Similar content being viewed by others
Notes
MedDRA®, the Medical Dictionary for Regulatory Activities terminology, is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). The MedDRA® trademark is owned by the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) on behalf of the ICH.
References
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–7.
Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816.
Antons KA, Williams CD, Baker SK, Phillips PS. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119(5):400–9.
Ronaldson KJ, O’Shea JM, Boyd IW. Risk factors for rhabdomyolysis with simvastatin and atorvastatin. Drug Saf. 2006;29(11):1061–7.
Ardati A, Stolley P, Knapp DE, Wolfe SM, Lurie P. Statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2005;14(4):287.
Eriksson M, Angelin B, Sjoberg S. Risk for fatal statin-induced rhabdomyolysis as a consequence of misinterpretation of ‘evidence-based medicine’. J Intern Med. 2005;257(3):313–4.
Parale GP, Baheti NN, Kulkarni PM, Panchal NV. Effects of atorvastatin on higher functions. Eur J Clin Pharmacol. 2006;62(4):259–65.
Tatley M, Savage R. Psychiatric adverse reactions with statins, fibrates and ezetimibe: implications for the use of lipid-lowering agents. Drug Saf. 2007;30(3):195–201.
Suribhatla S, Dennis MS, Potter JF. A study of statin use in the prevention of cognitive impairment of vascular origin in the UK. J Neurol Sci. 2005;229–230:147–50.
Golomb BA, Kane T, Dimsdale JE. Severe irritability associated with statin cholesterol-lowering drugs. QJM. 2004;97(4):229–35.
MHRA public assessment report. Statins: updates to product safety information. http://www.mhra.gov.uk/home/groups/pl-p/documents/websiteresources/n062559.pdf2009.
Ali AK. Pharmacovigilance analysis of adverse event reports for aliskiren hemifumarate, a first-in-class direct renin inhibitor. Ther Clin Risk Manag. 2011;7:337–44.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2009;32(1):19–31.
Rodriguez EM, Staffa JA, Graham DJ. The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf. 2001;10(5):407–10.
Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med. 2005;165(12):1363–9.
van Puijenbroek EP, Bate A, Leufkens HG, Lindquist M, Orre R, Egberts AC. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 2002;11(1):3–10.
Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315–21.
Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2013;10(7):796–803.
Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJ, Yuen N. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82(2):157–66.
Hallas J. Evidence of depression provoked by cardiovascular medication: a prescription sequence symmetry analysis. Epidemiology. 1996;7(5):478–84.
Tsiropoulos I, Andersen M, Hallas J. Adverse events with use of antiepileptic drugs: a prescription and event symmetry analysis. Pharmacoepidemiol Drug Saf. 2009;18(6):483–91.
Hallas J, Gaist D, Bjerrum L. The waiting time distribution as a graphical approach to epidemiologic measures of drug utilization. Epidemiology. 1997;8(6):666–70.
Morris JA, Gardner MJ. Calculating confidence intervals for relative risks (odds ratios) and standardised ratios and rates. Br Med J (Clin Res Ed). 1988;296(6632):1313–6.
Tuccori M, Lapi F, Testi A, Coli D, Moretti U, Vannacci A, et al. Statin-associated psychiatric adverse events: a case/non-case evaluation of an Italian database of spontaneous adverse drug reaction reporting. Drug Saf. 2008;31(12):1115–23.
Saheki A, Terasaki T, Tamai I, Tsuji A. In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm Res. 1994;11(2):305–11.
Galatti L, Polimeni G, Salvo F, Romani M, Sessa A, Spina E. Short-term memory loss associated with rosuvastatin. Pharmacotherapy. 2006;26(8):1190–2.
King DS, Wilburn AJ, Wofford MR, Harrell TK, Lindley BJ, Jones DW. Cognitive impairment associated with atorvastatin and simvastatin. Pharmacotherapy. 2003;23(12):1663–7.
Padala KP, Padala PR, Potter JF. Simvastatin-induced decline in cognition. Ann Pharmacother. 2006;40(10):1880–3.
Roth T, Richardson GR, Sullivan JP, Lee RM, Merlotti L, Roehrs T. Comparative effects of pravastatin and lovastatin on nighttime sleep and daytime performance. Clin Cardiol. 1992;15(6):426–32.
Schaefer EJ. HMG-CoA reductase inhibitors for hypercholesterolemia. N Engl J Med. 1988;319:1222–3.
Vgontzas AN, Kales A, Bixler EO, Manfredi RL, Tyson KL. Effects of lovastatin and pravastatin on sleep efficiency and sleep stages. Clin Pharmacol Ther. 1991;50(6):730–7.
Barth JD, Kruisbrink OA, Van Dijk AL. Inhibitors of hydroxymethylglutaryl coenzyme A reductase for treating hypercholesterolaemia. BMJ. 1990;301(6753):669.
Ehrenberg BL, Lamon-Fava S, Corbett KE, McNamara JR, Dallal GE, Schaefer EJ. Comparison of the effects of pravastatin and lovastatin on sleep disturbance in hypercholesterolemic subjects. Sleep. 1999;22(1):117–21.
Tuccori M, Montagnani S, Mantarro S, Capogrosso-Sansone A, Ruggiero E, Saporiti A, et al. Neuropsychiatric adverse events associated with statins: epidemiology, pathophysiology, prevention and management. CNS Drugs. 2014;28(3):249–72.
Engelberg H. Low serum cholesterol and suicide. Lancet. 1992;339(8795):727–9.
Liu X, Uchiyama M, Kim K, Okawa M, Shibui K, Kudo Y, et al. Sleep loss and daytime sleepiness in the general adult population of Japan. Psychiatry Res. 2000;93(1):1–11.
Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Arch Gen Psychiatry. 1985;42(3):225–32.
Kuppermann M, Lubeck DP, Mazonson PD, Patrick DL, Stewart AL, Buesching DP, et al. Sleep problems and their correlates in a working population. J Gen Intern Med. 1995;10(1):25–32.
Simon GE, VonKorff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154(10):1417–23.
Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22(Suppl. 2):S347–53.
Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262(11):1479–84.
Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population: influence of previous complaints of insomnia. Arch Intern Med. 1992;152(8):1634–7.
Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–36.
Motola D, Piccinni C, Biagi C, Raschi E, Marra A, Marchesini G, et al. Cardiovascular, ocular and bone adverse reactions associated with thiazolidinediones: a disproportionality analysis of the US FDA adverse event reporting system database. Drug Saf. 2012;35(4):315–23.
Sommet A, Grolleau S, Bagheri H, Lapeyre-Mestre M, Montastruc JL. Was the thrombotic risk of rofecoxib predictable from the French Pharmacovigilance Database before 30 September 2004? Eur J Clin Pharmacol. 2008;64(8):829–34.
Lindquist M, Stahl M, Bate A, Edwards IR, Meyboom RH. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf. 2000;23(6):533–42.
Tamura T, Sakaeda T, Kadoyama K, Okuno Y. Aspirin- and clopidogrel-associated bleeding complications: data mining of the public version of the FDA Adverse Event Reporting System, AERS. Int J Med Sci. 2012;9(6):441–6.
Murakami H, Sakaeda T, Kadoyama K, Okuno Y. Gender effects on statin-associated muscular adverse events: an analysis of the FDA AERS database. Pharmacol Pharmacy. 2013;4:340–6.
Moore N, Kreft-Jais C, Haramburu F, Noblet C, Andrejak M, Ollagnier M, et al. Reports of hypoglycaemia associated with the use of ACE inhibitors and other drugs: a case/non-case study in the French pharmacovigilance system database. Br J Clin Pharmacol. 1997;44(5):513–8.
Poluzzi E, Raschi E, Moretti U, De Ponti F. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS). Pharmacoepidemiol Drug Saf. 2009;18(6):512–8.
Poluzzi E, Raschi E, Motola D, Moretti U, De Ponti F. Antimicrobials and the risk of torsades de pointes: the contribution from data mining of the US FDA Adverse Event Reporting System. Drug Saf. 2010;33(4):303–14.
Wahab IA, Pratt NL, Wiese MD, Kalisch LM, Roughead EE. The validity of sequence symmetry analysis (SSA) for adverse drug reaction signal detection. Pharmacoepidemiol Drug Saf. 2013;22(5):496–502.
Lai EC, Yang YH, Lin SJ, Hsieh CY. Use of antiepileptic drugs and risk of hypothyroidism. Pharmacoepidemiol Drug Saf. 2013;22(10):1071–9.
Acknowledgments
We thank the Japan Medical Information Research Institute, Inc. for providing the database of prescriptions.
Conflict of interest statement
No sources of funding were used to assist in the preparation of this study. Mitsutaka Takada, Mai Fujimoto, Kohei Yamazaki, Masashi Takamoto, and Kouichi Hosomi have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted in the Division of Clinical Drug Informatics, School of Pharmacy, Kinki University, Higashi-osaka, Osaka, Japan.
Rights and permissions
About this article
Cite this article
Takada, M., Fujimoto, M., Yamazaki, K. et al. Association of Statin Use with Sleep Disturbances: Data Mining of a Spontaneous Reporting Database and a Prescription Database. Drug Saf 37, 421–431 (2014). https://doi.org/10.1007/s40264-014-0163-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-014-0163-x