Abstract
Since the 1970s, clinicians have increasingly become more familiar with hyperprolactinemia (HPRL) as a common adverse effect of antipsychotic medication, which remains the cornerstone of pharmacological treatment for patients with schizophrenia. Although treatment with second-generation antipsychotics (SGAs) as a group is, compared with use of the first-generation antipsychotics, associated with lower prolactin (PRL) plasma levels, the detailed effects on plasma PRL levels for each of these compounds in reports often remain incomplete or inaccurate. Moreover, at this moment, no review has been published about the effect of the newly approved antipsychotics asenapine, iloperidone and lurasidone on PRL levels. The objective of this review is to describe PRL physiology; PRL measurement; diagnosis, causes, consequences and mechanisms of HPRL; incidence figures of (new-onset) HPRL with SGAs and newly approved antipsychotics in adolescent and adult patients; and revisit lingering questions regarding this hormone. A literature search, using the MEDLINE database (1966–December 2013), was conducted to identify relevant publications to report on the state of the art of HPRL and to summarize the available evidence with respect to the propensity of the SGAs and the newly approved antipsychotics to elevate PRL levels. Our review shows that although HPRL usually is defined as a sustained level of PRL above the laboratory upper limit of normal, limit values show some degree of variability in clinical reports, making the interpretation and comparison of data across studies difficult. Moreover, many reports do not provide much or any data detailing the measurement of PRL. Although the highest rates of HPRL are consistently reported in association with amisulpride, risperidone and paliperidone, while aripiprazole and quetiapine have the most favorable profile with respect to this outcome, all SGAs can induce PRL elevations, especially at the beginning of treatment, and have the potential to cause new-onset HPRL. Considering the PRL-elevating propensity of the newly approved antipsychotics, evidence seems to indicate these agents have a PRL profile comparable to that of clozapine (asenapine and iloperidone), ziprasidone and olanzapine (lurasidone). PRL elevations with antipsychotic medication generally are dose dependant. However, antipsychotics having a high potential for PRL elevation (amisulpride, risperidone and paliperidone) can have a profound impact on PRL levels even at relatively low doses, while PRL levels with antipsychotics having a minimal effect on PRL, in most cases, can remain unchanged (quetiapine) or reduce (aripiprazole) over all dosages. Although tolerance and decreases in PRL values after long-term administration of PRL-elevating antipsychotics can occur, the elevations, in most cases, remain above the upper limit of normal. PRL profiles of antipsychotics in children and adolescents seem to be the same as in adults. The hyperprolactinemic effects of antipsychotic medication are mostly correlated with their affinity for dopamine D2 receptors at the level of the anterior pituitary lactotrophs (and probably other neurotransmitter mechanisms) and their blood–brain barrier penetrating capability. Even though antipsychotics are the most common cause of pharmacologically induced HPRL, recent research has shown that HPRL can be pre-existing in a substantial portion of antipsychotic-naïve patients with first-episode psychosis or at-risk mental state.
Similar content being viewed by others
1 Prolactin (PRL)
Prolactin (PRL) (Lat. pro = for; lac, gen. lactis = milk), also called lactotrophin hormone, is a polypeptide hormone that is mainly synthesized and secreted in a pulsatile manner (with around 10 peaks per day in young adults) from lactotroph cells of the anterior lobe of the pituitary gland (i.e., the adenohypophysis) [1–6]. These cells comprise between 20 and 50 % of the cellular population of the gland [4, 5], with those in the more inner zones being more responsive to dopamine, a neurotransmitter playing a pivotal role in the regulation of PRL secretion. Lactotroph cells in the outer zone are more responsive to thyroid-releasing hormones, one of the other substances playing a role in PRL secretion [5]. However, although PRL is typically thought of as a pituitary-derived hormone, PRL secretion is not restricted to the pituitary gland [7]. Other organs and tissues in the body also produce PRL, including the hypothalamus, telencephalon, brain stem, spinal cord, choroid plexus, mammary gland, some immune cells and circumventricular organs [8, 9].
Prolactin, discovered more than 80 years ago [8], is composed of 199 amino acids, having a molecular weight of about 23 kDa [4–6, 8, 10–12]. It has been found to be involved in over 300 separate functions, which can be divided into the following categories: reproduction, water and electrolyte balance, growth and development, endocrinology and metabolism, brain and behavior and immunoregulation [12–14]. Its main physiological functions include the induction and maintenance of milk production, breast enlargement during pregnancy, inhibition of hypothalamic gonadotrophin-releasing hormone, and maintenance of proper ovarian function and of progesterone-secreting structures [3, 6, 12, 15–17]. Despite the fact that almost 300 functions could be identified for this hormone in various species, the question remains open as to which of them are really relevant in humans [8, 18].
2 Physiology
A large variety of stimuli, provided by the environment and the internal milieu (exercise, suckling, stress, sleep, meals, sexual intercourse, levels of ovarian steroids, etc.), are involved in the fine balance of stimulation and inhibition of PRL release. The secretion of PRL is under complex control of peptide and steroid hormones and neurotransmitters, which act as inhibitory [PRL-inhibiting factors (PIFs)] or stimulatory [PRL-releasing factors (PRFs)] factors by a direct effect on the lactotroph cells or by indirect pathways (modulators or indirect regulators) [17, 19–21].
The periventricular (periventricular nucleus and arcuate nucleus) and the medial (paraventricular nucleus and supraoptic nucleus) hypothalamic subdivisions are the major regions associated with PRL homeostatis [17, 19]. In the context of this review, two neurotransmitters are important to discuss: dopamine and serotonin (5-HT).
2.1 Role of Dopamine in PRL Secretion
Dopamine is the most important hypothalamic PIF [4]. It exerts a tonic inhibition on PRL secretion, mainly via two pathways: the tuberoinfundibular dopaminergic (TIDA) system and the tuberohypophysial tract [4, 17, 19–21]. The TIDA system, consisting of a population of dopaminergic neurons found in the arcuate nucleus of the hypothalamus, is the most important in regulating PRL release in humans. These dopaminergic neurons release dopamine into the perivascular spaces of the medial eminence. From here, dopamine is subsequently transported via long portal vessels to the anterior lobe of the pituitary [4, 17, 19–22]. The other inhibitory dopamine pathway, the tuberohypophysial tract, also originates in the arcuate nucleus and projects to the intermediary and posterior pituitary, and dopamine, released in the blood, reaches the lactotroph cells via the short portal vessels [4, 19, 21]. Dopamine binds to the dopamine D2 receptors (D2R) on the membrane of the lactotroph cells. D2R stimulation inhibits the PRL gene transcription, synthesis and release of PRL as well as lactotroph proliferation [4, 6, 11, 13, 17, 19, 21, 23–25]. The TIDA network is partially regulated by an autocrine negative feedback by PRL on its own release. An increase in circulating PRL levels results in higher activity of TIDA neurons, whereas a decrease in circulating PRL levels lowers their activity [4, 17]. As such, PRL regulates its own release by acting directly on the hypothalamic dopaminergic neurons, probably through regulating the activity of tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis [4, 22].
A blockade of D2R counteracts the tonic inhibitory effect on the PRL secretion [26]. Inhibition of the dopamine transmission, especially through D2R blockade on the lactotroph cells, results in disinhibition of PRL secretion: the stronger the dopamine blockade, the higher the PRL elevation [27]. Antipsychotics have a D2-blocking effect and can therefore elevate the secretion of PRL [28, 29]. Conversely, dopamine agonists (e.g., bromocriptine) suppress PRL secretion [23].
2.2 Role of Serotonin in PRL Secretion
While hyperprolactinemia (HPRL) is well recognized in relation to antipsychotic medication and its antidopaminergic activity, there is relatively little awareness of the impact of substances interfering with serotonergic neurotransmission on the PRL levels [21].
Drugs that increase extracellular levels of 5-HT or are direct agonists of 5-HT receptors elicit PRL secretion, and blocking 5-HT synthesis or transmission results in blunted PRL release [30–33].
5-HT has a stimulatory role in PRL secretion through a complex, multi-level action on both the hypothalamus and the pituitary [4]. Although some research has demonstrated a direct prosecretory effect on the anterior pituitary cells [34], 5-HT is an indirect modulator of PRL secretion, with the hypothalamus as its predominant site of action, where several 5-HT receptors types have been found to play a role in PRL secretion [17, 19, 21, 35, 36].
The serotonergic pathways involved in the regulation of PRL secretion originate from cells in the dorsal raphe nucleus and terminate in the paraventricular nucleus of the hypothalamus, exerting their action via 5-HT1A and 5-HT2A/C receptors (5-HT2A/CR) [21, 35, 37]. The paraventricular nucleus contains cells producing oxytocin and vasoactive intestinal peptide (VIP), both considered to be PRFs [21]. The 5-HT-mediated PRL increase is probably mediated via stimulation of PRFs, of which oxytocin and VIP are the best studied [17, 21]. The oxytocin cells project to the posterior lobe of the pituitary, and oxytocin reaches the anterior pituitary via portal vessels as well as through the systemic circulation, and stimulates the lactotrophs via their oxytocin receptors, causing PRL release [38, 39]. The VIP cells project to the anterior pituitary, where VIP binds to the receptor on the lactotroph cell membrane, stimulating PRL release [40]. Moreover, VIP may also be produced in the pituitary itself, stimulating PRL release by autocrine and paracrine mechanisms [17]. Furthermore, animal studies suggest a link between oxytocin and VIP action on PRL release [41]. Although pharmacological and anatomic data indicate that the paraventricular nucleus represents a major regulating site of 5-HT-induced PRL release, ablation of the paraventricular nucleus does not entirely abolish the PRL response to 5-HT agonists, demonstrating that other pathways also contribute to 5-HT-induced PRL release [42, 43]. An alternate path for 5-HT-induced PRL release is inhibition of the TIDA dopamine cells. However, there is little synaptic contact between 5-HT fibers and TIDA cells, indicating that if direct inhibition of dopamine cells occurs by 5-HT, it is through volume transmission of 5-HT in this region [44]. There is more direct evidence for 5-HT stimulation of GABA-ergic neurons in the vicinity of TIDA cells. Stimulation of these GABA-ergic interneurons will result in an inhibition of TIDA neurons, releasing the tonic inhibition of PRL secretion [17, 21, 45].
2.3 Role of Other Substances in PRL Secretion
As already indicated above, PRL secretion is under the complex control of peptide and steroid hormones and neurotransmitters, which can act as inhibitory or stimulatory factors. Somatostatin, acetylcholine, endothelins, norepinephrine, growth hormone, etc. are identified as PIFs. Thyrotropin-releasing hormone (TRH) as well as angiotensin II, vasopressin, galanin, etc. stimulate PRL secretion [17, 19, 21, 46]. Estrogen is a physiological regulator of PRL synthesis in pituitary lactotroph cells [47, 48]. Estrogens can increase PRL secretion in different ways: through an augmentation of the number of PRL-secreting lactotroph cells in the pituitary gland; through an elevation of the sensitivity to TRH; through a decrease in the number of pituitary dopamine receptors; and through an upregulation of the expression of the PRL receptor gene [6, 30]. Veselinović et al. [49] showed that estrogen treatment in healthy volunteers sensitizes women for PRL-elevating properties of antipsychotic medication. It is also known that ghrelin, a peptide hormone involved in metabolic homeostasis, stimulates PRL secretion, most probably by a direct action on the pituitary somatomammotroph cells (progenitor cells of lactotroph cells that can produce both PRL and growth hormone) [50–52]. A relatively large body of evidence has shown that tachykinins can modify the secretion of PRL in a rather complex fashion, having a stimulatory effect at the pituitary level, but in some circumstances (via modulating hypothalamic dopamine release) also an inhibitory one [4]. Finally, there is some evidence to suggest that endogenous opioids play an important role in regulating PRL secretion, especially in relation to stressful stimuli. Most stressful stimuli lead to a reduction in the activity of TIDA neurons through activation of inhibitory neuronal pathways, with a consequent increase in PRL secretion [4].
3 Diagnosis of Hyperprolactinemia (HPRL)
3.1 Normal and Elevated PRL Values
Normal serum concentration of PRL varies with sex [11, 16, 53]. Hence, normal PRL levels for men and women are different, with ranges of 10–20 and 10–25 ng/ml, respectively [54, 55]. There are also marked interindividual differences; normal PRL serum levels differ appreciably from one individual to another [17, 46, 56, 57]. Serum PRL also displays pronounced circadian variations (up to four times), with maximal concentrations occurring during sleep [5, 11, 12, 15, 16, 53, 56], with a peak of up to 30 ng/ml [5, 6] between 4 a.m. and 6 a.m. [6, 55], reaching a nadir during waking hours [5]. During sleep, PRL secretion is highest during REM sleep. PRL levels are also subject to seasonal changes (higher in the spring and summer) [5, 58, 59]. An increased release of PRL occurs during pregnancy (up to a maximum of 200 ng/ml) and breastfeeding (up to a maximum of 300 ng/ml) [6, 15, 16, 23, 46]. Although PRL is not commonly recognized as a hormone which changes significantly within the menstrual cycle or after menopause, it has been found that PRL levels vary significantly throughout the menstrual cycle [60]. These levels are higher during the menstrual mid-cycle (particularly the second half of the cycle) [61], compared with follicular and luteal phases. It also varies significantly between pre- and postmenopausal women (higher in premenopausal women) [60]. Therefore, the utility and accuracy of PRL testing may be improved by applying specific reference intervals for each phase of the menstrual cycle, with the recommendation that PRL levels should only be measured in the follicular phase, well before the mid-cycle [60]. Whether there is a change in PRL with the onset of menopause remains controversial. In healthy normoprolactinemic subjects, both increases and decreases in PRL have been reported [62]. Finally, serum PRL rises after exercise, meals, sexual intercourse, minor surgical procedures, general anesthesia, acute myocardial infarction, an epileptic seizure (including after electroconvulsive therapy) and other forms of acute stress [5, 6, 55, 61]. Interestingly, several recent studies reported that current and ex-cigarette smokers on antipsychotic medication have significantly lower mean PRL levels, compared with nonsmokers [63–65]. It is known that cigarette smoking is a potent inducer of the hepatic cytochrome P450 1A2 enzyme, and smoking may therefore result in a reduction of serum concentration of some antipsychotic drugs, which may, in turn, result in lower PRL levels [63]. However, further studies are needed to elucidate the underlying mechanism(s) [63, 64].
Hyperprolactinemia is usually simply defined as a sustained level of PRL above the laboratory upper level of normal [5, 66, 67]. However, pathological HPRL has to be defined as sustained circulating PRL levels above normal range in conditions other than, for example, pregnancy and lactation, when physiological HPRL occurs [8]. Although the clinician/researcher needs to be aware that laboratory ranges may differ between sites [67], in most laboratories and according to the most recent and more conservative reports, the upper limits for PRL serum concentrations are set at 20 ng/ml for men and 24–25 ng/ml for non-pregnant, non-nursing women [68]. Therefore, HPRL is usually defined as fasting levels at least 2 h after waking above 20 ng/ml in men, and above 25 ng/ml in women [20]. However, these limit values show some degree of variability in clinical trial reports (or, in many reports, are simply are not stated), making the interpretation and comparison of data across studies difficult. Reviewing numerous studies, we see that limit values are set between 11 ng/ml (for men) and 50 ng/ml (for women) (see Table 1). Some laboratories also give different ranges for pre- or postmenopausal women.
Prolactin-secreting pituitary adenomas (prolactinomas) (about 40 % of pituitary tumors produce PRL) [6] are the most common cause of PRL levels greater than 100 ng/ml. Thus, pituitary tumors are an important consideration for antipsychotic-treated patients with levels above 95–118 ng/ml [67, 115]. PRL levels above 200 ng/ml almost always indicate the presence of a lactotroph adenoma [55]. PRL concentration also correlates with tumor size [5, 116]. A serum PRL level >200–250 ng/ml is usually due to a macroadenoma (>1 cm in diameter), rather than a microadenoma (<1 cm diameter) [116, 117]. In the event of a macroprolactinoma, the PRL concentration can even rise to values as high as 35,000 ng/ml (see Table 2). However, it is important to note that PRL is not a perfect tumor marker. There have been reports of tumor growth of lactotroph cells in the absence of increasing PRL levels [116–118]. Therefore, the monitoring of PRL levels alone is not adequate to exclude an expanding tumor mass [116]. Moreover, PRL-producing tumors exist “silently” (a clinically insignificant adenoma) in up to 5–10 % of the adult population [119]. Elevated PRL levels, but <100 ng/ml, are most commonly due to a medication effect [55], although increases beyond 300 ng/ml with an antipsychotic medication have been reported [67, 115].
Although some case reports have noted the resolution of prolactinoma after switching from a “PRL-raising” to a “PRL-sparing” antipsychotic [120, 121], and some studies suggest that certain “PRL-raising” antipsychotics may have a causal relationship with pituitary adenoma [122], this issue remains controversial [123]. No literature exists that establishes a causal relationship between antipsychotics and pituitary tumors, or demonstrates that HPRL can cause a pituitary adenoma, including in normal physiological states of HPRL, such as pregnancy [124]. Moreover, investigation and reporting biases may have influenced the disproportional reporting of pituitary tumor in patients on “PRL-raising” antipsychotics [125]. As there are no prospective studies or randomized trials available, this area needs further research.
Values that fall within the interval of high PRL levels can bring about clinically significant symptoms [23]. Serri et al. [126] provide some rules of thumb regarding PRL levels and clinical presentations in premenopausal women: marked PRL excess (>100 ng/ml) is commonly associated with hypogonadism, galactorrhea and amenorrhea; moderate PRL excess (51–75 ng/ml) is associated with oligomenorrhea; mild PRL excess (31–50 ng/ml) is associated with short luteal phase, decreased libido and infertility. These breakpoints help to place some of the more commonly observed elevations in plasma PRL in clinical perspective [55]. However, increased PRL levels can occur without clinical symptoms [127]. Although it has been suggested that increased PRL levels are believed to be responsible for sexual impairment, one is struck by the fact that the evidence is contradictory and inconclusive [128, 129]. Some studies have shown an association between sexual side effects and PRL elevation, while others have failed to support this [129]. It is therefore important to distinguish between symptomatic and asymptomatic HPRL, although this distinction is seldom made in the scientific literature [55].
3.2 Measurement of PRL Levels
Despite the well-recognized variability in PRL levels, most reports do not provide much or any data detailing the measurement of PRL levels (e.g., the time of sampling). These may be relevant factors in individual patients who have borderline raised levels. A complicating factor during measurement of PRL levels is the presence of macroprolactinFootnote 1, which is essentially biologically inactive, but may lead to falsely high PRL levels as measured by many assays [66]. Conservative estimates suggest that the presence of macroprolactin leads to misdiagnosis in as many as 10 % of all reported instances of biochemical HPRL [90]. Macroprolactinemia can, however, be revealed by polyethylene glycol precipitation of serum samples [17, 90]. Thus, awareness and understanding of the potential impact of factors that affect PRL are important in the interpretation of PRL testing results [16]. When assessing PRL levels other points to be aware of are [130]:
-
All serum measurements must been done by the same laboratory.
-
All bloods must have been drawn in the morning with the patient in a fasting state.
-
To confirm diagnosis, at least one repeated measurement is required after the initial elevated level.
There is a variety of reported units of measurement of PRL levels. Serum PRL concentrations are usually expressed in units of either ng/ml (or the equivalent μg/l), nmol/l, pmol/l, or mIU/l [5, 6]. US data are often presented in ng/ml, whereas most UK and EU data are in mIU/l [5]. Most clinical papers do not state the conversion factor needed, while there exists variability in conversion factors that are reported. In general terms, ng/ml × 21.2 converts to mIU/l (although some conversion factors given are as high as 36) [5, 66, 67, 115], ng/ml × 43.478 converts to pmol/l [70], and ng/ml × 0.043 converts to nmol/l [6].
The reporting of PRL levels remains poor in many published studies. A baseline plasma PRL level and at least one follow-up measurement should be reported. However, several follow-up measurements over a longer (≥1 year) study period are preferable as continuity and longer-term data are needed. Moreover, there has been a tendency to report mainly mean PRL values or mean change PRL data that do not easy allow interpretation of the precise number of patients developing HPRL or the clinical significance of these elevations. Not to report categorical data (thus not telling explicitly how many patients have HPRL) makes it difficult for clinicians to advise their patients on the appropriate risk with a particular antipsychotic compound [131]. Therefore, categorical outcomes for abnormal PRL levels need to be reported, as well as outcomes on the severity of the HPRL in individual patients. PRL levels should also be measured by a morning, fasting, pre-medication sample. Lastly, it is also important to mention whether patients at baseline were switched from another antipsychotic or not, as well as to provide the thresholds used for the registered (ab)normal plasma PRL values.
3.3 Incidence of HPRL
Hyperprolactinemia is one of the most common endocrinological disorders of the hypothalamic-pituitary axis [17]. The estimated incidence of HPRL (serum PRL values >25 ng/ml) in the non-selected healthy population is low (0.4 %) [13]. However, this estimate may be too low, given that autopsy reports state that more than 10 % of the population have a pituitary tumor [14].
Considering PRL responses to antipsychotic medication, it is repeatedly found that PRL responses to these medications are greater in females than in males [49, 107, 132–141]. This difference can be explained by the ability of estrogens to elevate serum PRL levels and enhance responsiveness to PRL-releasing stimuli [49, 142]; estrogens increase the number of lactotrophic cells of the anterior pituitary and act on the hypothalamus to decrease dopamine content [49]. Among male patients, age was found to have no influence on PRL concentrations, whereas in women, a younger age was associated with higher PRL levels, as expected for their reproductive status [105, 143]; women of reproductive age experience raised PRL secondary to antipsychotic medication more frequently than postmenopausal women [107]. Although HPRL is most established in adult patients, it can be of clinical significance in children and adolescents as well [144–154]. Once again, post-pubertal girls may be more sensitive to this adverse effect, given that estrogen stimulates PRL synthesis and enhances PRL responses to antipsychotic medication [155].
4 Causes of Elevated PRL Values and HPRL
5 Methods
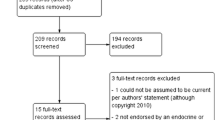
To get a fuller picture of the relationship between antipsychotics and PRL elevation and because, at this moment, no review has been published about the effect of the newly approved antipsychotics asenapine, iloperidone and lurasidone on PRL levels, a literature search (1966 until December 2013), using the MEDLINE database, was conducted for English-language published reviews, meta-analyses and clinical trials of first-generation antipsychotics (FGAs), second-generation antipsychotics (SGAs) and newly approved antipsychotics. The following keywords were used: “antipsychotics,” “neuroleptics,” “clozapine,” “olanzapine,” “quetiapine,” “amisulpride,” “ziprasidone,” “aripiprazole,” “risperidone,” “sertindole,” “paliperidone (extended-release and palmitate),” “asenapine,” “iloperidone,” “lurasidone,” “schizophrenia,” “prolactin” and “hyperprolactinemia”. The review is based upon studies carried out with adults as well as children and adolescents. We attempted to identify additional studies through searches of the reference lists of identified studies and reviews.
6 PRL and Antipsychotics
6.1 General Remarks
After the introduction of chlorpromazine into clinical practice more than half a century ago, antipsychotic medication rapidly became the cornerstone of pharmacological treatment for schizophrenia. Antipsychotic drugs are arbitrarily divided into first-generation and second-generation agents (including the newly approved antipsychotics). Although most antipsychotic drugs are now used to treat a broad range of symptoms and disorders, their primary indication remains psychotic disorder, more specifically schizophrenia and schizophrenia-related disorders.
It was accepted for a long time that serum PRL concentrations are not elevated in untreated schizophrenic patients, unless they have other underlying pathologies (e.g., prolactinoma) [12, 13, 160]. Several studies [138, 161–165] showed that PRL values in untreated schizophrenic patients were comparable to those of healthy control subjects. This finding would indicate that the high PRL values observed in schizophrenic patients treated with antipsychotic medication have to be attributed to the effects of this medication rather than to possible underlying effects of the disease itself [56]. The possibility that schizophrenia itself causes elevated PRL values seemed therefore excluded [14]. However, recent research showed that HPRL can be pre-existing in some patients with schizophrenia [166]. One of these studies on PRL levels in antipsychotic-free patients with schizophrenia found HPRL (>15.2 ng/ml in men and >23.3 ng/ml in women) in 23.8 % of antipsychotic-naïve patients with an at-risk mental state for psychosis and in 33.3 % of antipsychotic-naïve patients with first-episode psychosis [79]. This finding is in accordance with the results of the European First Episode Schizophrenia Trial (EUFEST) study, showing HPRL (>18 ng/ml in men and >25 ng/ml in women) in 71 % of first-episode patients of whom approximately half had never been exposed to antipsychotic treatment [93], as well as with other studies of drug-naïve patients [167–169]. The increased PRL concentrations in antipsychotic-naïve patients do not appear to be due to important confounding variables such as gender, smoking and body mass index (BMI), nor to the effects of elevated thyroid stimulating hormone (TSH), ghrelin, or cortisol [168]. Therefore, these findings suggest that HPRL in schizophrenia is not necessarily only caused by antipsychotic medication, but might already be present in antipsychotic-naïve first-episode patients and even in prodromal stages [79]. HPRL in these patients can be caused by general stress associated with the illness experience [79], or by a pre-existing vulnerability [168], including a genetic predisposition [170]. One genetic study suggested a possible abnormality of the functional −1149 G/T polymorphism of the PRL gene in schizophrenia, especially in male patients. Based on the results of this study, it may be speculated that individuals with a preponderance of the G allele of the polymorphism react more strongly to stress with a higher production of PRL [170].
The association between antipsychotic medication and HPRL has been under investigation since at least the 1970s [125]. Although HPRL can be found in association with all antipsychotic medications, these medications differ in their propensity to cause PRL elevation [171]. The highest rates of HPRL are reported in association with risperidone, amisulpride and sulpiride, often in as high as 80–90 % of all female subjects and consistently greater than with the FGAs and other SGAs (see 6.3.1.1 and 6.3.1.7). However, significant rates of HPRL of lesser severity and more transience have also been reported in association with several other antipsychotics [66]. On the basis of the scientific literature, a distinction has been made by numerous authors between antipsychotics with a “PRL-raising potential” (among which are counted the FGAs and the SGAs amisulpride, risperidone and paliperidone) and the “PRL-sparing” antipsychotics (clozapine, quetiapine, olanzapine, ziprasidone and aripiprazole) [46, 57, 105, 109, 140, 172–177]. Nevertheless, this terminology may incorrectly lead clinicians to conclude that antipsychotic medication such as clozapine, quetiapine and even aripiprazole can never be associated with significant HPRL. Thus, although this terminology can be helpful, it over-simplifies the picture [115]. When looking at individual studies, in order to make an adequate evaluation of the PRL-elevating propensity of antipsychotics, information on PRL baseline values and previous treatment is necessary.
A complex question relating to PRL levels concerns the persistence of HPRL [66]. With the exception of some studies (e.g., the study of Eberhard et al. [178] on risperidone; see 6.3.1.7), most of the reported PRL data are of too short duration to make definitive statements regarding this issue [179].
6.2 PRL and First-Generation Antipsychotics (FGAs)
Elevated PRL values are regularly observed when FGAs are used. In most patients, such values are an unavoidable side effect [12, 23]. FGAs induce a significant rise in serum concentrations of PRL, which are around two to three times higher than the reference values [53, 55, 56]. The PRL values that are noted with FGAs are in general lower than 100 ng/ml, although in some patients values of up to 365 ng/ml have been observed [29]. In a study by Montgomery et al. [105], HPRL (>18.4 ng/ml for men, >26 ng/ml for women, with a mean PRL level of 42.1 ng/ml) was found in 71 % of the patients treated with FGAs. In 37 % of the patients, PRL values exceeded twice the normal PRL values.
It has been suggested that patients receiving long-term neuroleptic treatment may develop tolerance to the secondary raising effect, with serum PRL concentration returning to normal with continued treatment. However, conflicting results have been reported. A few studies reported that tolerance can develop with time. Marken et al. [180], for example, found that 37.5 % of men and 27 % of women, who had been treated with FGAs for at least 5 years, had normal PRL levels. Brown and Laughren [181] found that after 4 months of treatment, PRL reached a stable level, substantially lower than that at the onset of FGAs treatment and not differing from that after years of treatment. Other studies, however, reported that tolerance does not occur. Meltzer and Fang [182, 183] studied serum PRL levels, before and during long-term administration of phenothiazines on a twice daily schedule, in 27 newly admitted schizophrenic patients. By 72 h after the initiation of treatment, all 27 patients had persistently elevated serum PRL levels, averaging a 3.2- and 3.8-fold increase in normal PRL values in men and women, respectively. Serum PRL levels remained elevated during the 1- to 3-month period subjects were studied, suggesting there was no tolerance to this effect of phenothiazines. A Japanese study failed to demonstrate tolerance in 27 chronic inpatients with schizophrenia receiving long-term haloperidol [184]. According to an overview of Meltzer [185] concerning long-term effects of FGAs on the neuroendocrine system, serum PRL levels remain elevated in most, but not all, schizophrenic patients receiving FGAs chronically, if the dose administered is high enough. Tolerance to these PRL elevations may develop in some subjects, but even their PRL levels, though still within normal limits, are higher than baseline levels.
6.3 PRL and Second-Generation Antipsychotics (SGAs)
6.3.1 PRL and SGAs in Adults
It has been established that, as a group, the SGAs cause a lesser elevation of the PRL plasma levels than the FGAs [90, 138, 186, 187]. Their greater specificity, resulting in a lesser blockade of the dopaminergic receptors, but also their stronger 5-HT2AR blockade, is thought to explain the more limited elevation of the PRL levels [15, 188] (see also 6.5). The notable exceptions in this regard are amisulpride, risperidone and paliperidone. However, with the exception of aripiprazole and clozapine, all SGAs have a standard warning regarding PRL elevations in their US product labels.
6.3.1.1 Amisulpride
Amisulpride, which is structurally related to the older high-PRL-elevating benzamide, sulpiride [11, 189], is associated with high rates of HPRL. Amisulpride also has a pronounced PRL-elevating effect which appears to be independent of dosage and duration of administration [190]. Even at relatively low doses (50 mg/day) amisulpride seems to induce substantially elevated PRL values [191–195], and brings about greater increases in PRL values than olanzapine and risperidone [193, 196, 197]. Kopecek et al. [193] found that subjects who receive doses as low as 50 mg/day have HPRL in almost all cases. This HPRL is significantly high (mean 113 ng/ml), and higher in females (160 ng/ml) than in males (48 ng/ml). Paparrigopoulos et al. [198] found a 100 % prevalence of HPRL for patients receiving amisulpride. A 12-month, open-label clinical trial with flexible doses of amisulpride (mean dose 501.4 ± 283.5 mg/day at month 12) found HPRL to be the most common (41.9 %) reported adverse effect among patients with schizophrenia. At baseline, the mean serum PRL level of all patients was 42.7 ng/ml, which increased significantly to 92.7 ng/ml at 8 weeks (p < 0.001) and then decreased to 73.3 ng/ml at month 12 [199]. Another 12-month trial found HPRL in 75.9 % of men and 85.7 % of women [200]. Frighi et al. [201], investigating the safety of antipsychotic medication in individuals with intellectual disability, found all participants on amisulpride/sulpiride had HPRL. Therefore, amisulpride is regarded to be the antipsychotic with the potential for maximum PRL elevation [66]. Nevertheless, HPRL rapidly reverses following amisulpride discontinuation [190, 195].
6.3.1.2 Aripiprazole
Aripiprazole belongs to the group of “PRL-sparing” antipsychotics [202–204]. In most studies with aripiprazole, PRL levels were found to decrease, even below those expected from placebo, or to remain unchanged over all dosages [83, 85, 86, 94, 108, 123, 200, 206–221]. However, new-onset HPRL with aripiprazole has been found [95, 108, 217]. In the study of McQuade et al. [217], among patients who had normal PRL levels at baseline, 8 % in the aripiprazole (15–30 mg/day) group experienced increases above the upper limit of normal (not defined) at some point during the trial. In general, HPRL prevalence rates of 3.1–9 % seem consistent [108, 191, 214, 217, 220–222], but can be sometimes higher. For example, Kwon et al. [83] found 10.7 % of patients to have an abnormal PRL level (>23 ng/ml) throughout the entire aripiprazole (10–30 mg/day) study (26 weeks). In another 26-week trial of aripiprazole (mean daily dose at endpoint 18.7 mg) versus “standard of care” agents (olanzapine, quetiapine or risperidone; mean daily dose at endpoint 12.5 mg, 386.8, and 4.6 mg, respectively), Kerwin et al. [223] found 16.8 % of aripiprazole-treated patients had potentially clinically relevant PRL elevations (compared with 54.4 % in the “standard of care” group). However, what is meant with “potentially clinically relevant PRL elevations” is not stated by the authors. Byerly et al. [224] showed that aripiprazole in combination with risperidone or olanzapine significantly reduced the PRL levels induced by these antipsychotics during the first week of treatment. In combination with risperidone, aripiprazole even decreased PRL levels to within the normal range of PRL values. These results are confirmed by other reports [86, 94, 216, 225–230]. The addition of aripiprazole 5 mg daily to long-acting risperidone was equally associated with a significant decrease in PRL levels. In an open, uncontrolled clinical trial with 13 patients with a severe mental disorder (schizophrenia and other unspecified psychoses), 12 patients showed a decrease in serum PRL levels (81 ± 46 μg/l at baseline vs. 42 ± 21 μg/l at month 1, p < 0.001, 52 % mean reduction) [231]. In two patients treated with adjunctive aripiprazole, PRL levels reverted to normality [231]. A recent meta-analysis of five randomized controlled trials (n = 639) comparing the safety and efficacy of adjunctive aripiprazole versus placebo for antipsychotic-induced HPRL found adjunctive aripiprazole to be both safe and effective as a reasonable choice treatment for patients with antipsychotic-induced HPRL. Adjunctive aripiprazole was associated with a 79.11 % (125/158) PRL level normalization rate. The appropriate dose of adjunctive aripiprazole may be 5 mg/day [232]. However, the add-on effects of aripiprazole in reversing antipsychotic-induced HPRL depend on the pharmacological properties of the pre-existing antipsychotic; adjunctive aripiprazole treatment reverses effectively HPRL induced by risperidone and olanzapine, but seems to be less effective for that induced by benzamide antipsychotics (amisulpride and sulpiride) [226, 233].
The absence of elevated PRL levels with aripiprazole can be accounted for by the partial agonism of aripiprazole with reference to the D2R [66, 234], most likely because it maintains a low level of activation of pituitary D2R [235]. As a partial D2R agonist, aripiprazole may be the drug of choice in patients suffering from both psychosis and prolactinoma [121].
Some data indicate that switching to aripiprazole also may be useful for resolving antipsychotic-induced HPRL [86, 94, 216]. Even rapid decreases of PRL levels seem to be achieved with aripiprazole switching strategies [86]. Lu et al. [94] assessed the time course of changes in antipsychotic-induced HPRL during the process of antipsychotic switching to aripiprazole (mean dose 18.5 mg/day). Twenty-three female schizophrenic subjects with risperidone (mean dose 4.8 mg/day)- or sulpiride (mean dose 500 mg/day)-induced symptomatic HPRL were recruited into the study. Serum PRL levels were measured at baseline, during the combination treatment period, and 4 weeks after having completed discontinuation of the pre-existing antipsychotic treatment. Switching antipsychotic drugs to aripiprazole was effective in reducing serum PRL levels in schizophrenic patients who received the PRL-raising antipsychotics. Mean serum PRL levels at baseline, during combination period, and after the switch were 97.0 ng/ml, 27.2 ng/ml (p < 0.001, vs. baseline) and 12.2 ng/ml (p < 0.001, vs. baseline), respectively. In a post hoc sub-analysis of an 8-week, open-label study in outpatients with schizophrenia (n = 269), Byerly et al. [86] examined short-term effects on PRL levels during a switch from risperidone (n = 105) or olanzapine (n = 164) to aripiprazole 30 mg/day with three switching strategies (I, immediate aripiprazole initiation with simultaneous immediate discontinuation of olanzapine/risperidone; II, immediate aripiprazole initiation while tapering off olanzapine/risperidone over 14 days; III, titrating aripiprazole upwards while tapering off olanzapine/risperidone over 14 days). Mean baseline PRL levels (ng/ml) were within normal range for the three olanzapine groups (group I, 11.7; group II, 13.2; group III, 11.2), but above normal for the risperidone groups (group I 39.7; group II 48.5; group III 33.5). Following aripiprazole initiation, mean PRL levels decreased significantly (p < 0.001) at week 1 and were maintained to week 8 in all groups irrespective of prior treatment. Previously elevated PRL levels in the risperidone groups were reduced to within normal range within 1 week, irrespective of switching strategy.
6.3.1.3 Clozapine
Clozapine induces little or no PRL elevation [11, 15, 30, 46, 110, 131, 155, 236–240] or is associated with significant decreases of PRL levels (after switching from a PRL-elevating FGA) [102, 241]. For example, Breier et al. [241] found that, after a baseline fluphenazine treatment period, clozapine (mean dose 403.6 mg/day) significantly decreased PRL levels from 53.3 ng/ml at baseline to 12.2 ng/ml at week 6. Long-term treatment with clozapine seems not to influence PRL levels significantly. One study showed that even after a treatment duration of 36 months with clozapine (median dose 300 mg), PRL levels remain low (mean 9 ng/ml) [242]. Despite all these observations, several studies indicated that clozapine can cause a brief (a few hours) elevation of PRL levels immediately after the dose is administered [11, 15, 238, 242], or can even be associated with HPRL [105, 243]. Turrone et al. [242] established that clozapine caused a doubling of the PRL baseline values in the 1–5-h period after medication administration. Montgomery et al. [105], who analyzed the data of three electronic databases (n = 422), determined that 11 % of the patients treated with clozapine had HPRL (>18.4 ng/ml for men, >26 ng/ml for women). However, the authors did not specify the mean treatment time of clozapine. They only noted that treatment in these chronically mentally ill patients had been stable for at least 1 month prior to the PRL assay. Bushe and Shaw [243] reported a prevalence of HPRL of 5 % with clozapine.
6.3.1.4 Olanzapine
Olanzapine, an antipsychotic with an intermediary D2R binding affinity, induces a moderate elevation of PRL levels [200, 244]. Categorical data show prevalence rates between 6 and 60 % [66, 74, 76, 99, 105, 114, 191, 245, 246]. Administration of olanzapine causes a doubling of PRL baseline values 6 h after it is taken [242].
Although switching from a PRL-raising antipsychotic to olanzapine can lead to a significant reduction [90, 247–249], even to the upper limit of normal [99], or a normalization of PRL levels [81, 205] in approximately half up to all of the patients who had elevated PRL levels at baseline, PRL levels during treatment with olanzapine remain raised in another substantial proportion (one-third to more than a half) of patients [205, 245, 246]. Moreover, olanzapine treatment can lead to new-onset HPRL in patients with normal PRL levels at baseline [87, 205].
Although Karagianis and Baksh [250], in a high-dose study (n = 24) on olanzapine, found no significant correlation between mean PRL values and olanzapine high-dose treatment groups (20, 25, 30 and 40 mg/day, mean duration of olanzapine therapy 15.3 months), most studies demonstrated a dose-dependent effect of oral olanzapine on the plasma PRL level of patients with schizophrenia, with higher doses associated with higher PRL levels [82, 102, 114, 251–253]. Significant dose-associated changes have also been identified with olanzapine long-acting injection [254].
Genetic polymorphisms may be associated with differences in PRL secretion mediated by SGAs. Cabaleiro et al. [255] found that some polymorphisms in D2R and 5-HTR2A are involved in PRL levels after administration of olanzapine. These results agree with those obtained in an earlier study revealing that PRL secretion induced by olanzapine (and risperidone) in healthy volunteers was modulated by Taq1A polymorphism of D2R. However, this factor was not linked to PRL secretion in quetiapine-treated healthy volunteers [256].
6.3.1.5 Paliperidone
Paliperidone extended-release (ER), the oral formulation, and paliperidone palmitate, the long-acting injectable formulation of paliperidone, frequently elevate PRL levels over both short- and longer-term treatment periods [257–259]. As paliperidone is the major active metabolite of risperidone, the mechanism of PRL elevation for paliperidone is likely similar to that of risperidone [260–262]. However, two recently published studies suggest that PRL levels seem to decrease after switching from risperidone to paliperidone in patients with a psychotic disorder [263, 264]. An analysis of abnormal PRL values in ten randomized clinical trials, including 3,173 patients treated with paliperidone palmitate, showed that, overall, at any time, PRL levels were elevated for 38.8 % of the subjects [265]. In general, plasma PRL levels are elevated to a greater extent in female than in male patients [77, 78, 91, 92, 257, 266–281], remain mostly elevated throughout treatment [257] and increases with increasing paliperidone ER dosage [257, 267, 269, 272, 276–279, 281, 282]. An analysis from a separate 6-day phase I study in stable subjects with schizophrenia found similar PRL pharmacokinetic profiles (maximum plasma concentration, C max and area under the curve, AUC) when subjects received the highest recommended dose of paliperidone ER (12 mg/day) compared with an average dose of risperidone (4 mg/day) [283].
6.3.1.6 Quetiapine
Research has shown that quetiapine, like clozapine, induces virtually no elevation of PRL in the blood [11, 15, 27, 46, 56, 104, 200, 245, 284–286]. Moreover, many studies show that PRL levels, after switching, decrease [113, 287–291], even to normal values [81, 100, 292–294], during treatment with quetiapine. However, once again, it would be a mistake to claim that quetiapine (like clozapine) can not cause an elevation of PRL values or induce HPRL. Some data indicate that quetiapine can bring about a transient rise in PRL levels [11, 15, 30, 242]. Kapur et al. [295] showed that in some patients, 2–3 h after a single dose, quetiapine (400–450 mg) corresponded with a transiently high D2R occupancy at around 60 % and a transiently elevated PRL level of 19–28 ng/ml. These PRL values returned to normal within 24 h. In studies that report categorical rates of HPRL, low prevalence rates in association with quetiapine have been reported, ranging from 0 to 29 % [66, 104, 105, 154, 191, 296]. Montgomery et al. [105] established that 22 % of the patients treated with quetiapine presented HPRL (>18.4 ng/ml for men, >26 ng/ml for women).
6.3.1.7 Risperidone
Risperidone has a high potential for PRL elevation. It causes more marked elevations in PRL than any other SGA [17, 155, 200, 297, 298]. There is also evidence to suggest that the effect of risperidone (as well as paliperidone) on PRL may exceed that of FGAs [115, 123] (with the exception of sulpiride) [299]. A number of different comparative PRL data sets find consistently that risperidone elevated PRL more commonly and to the same or a greater degree than haloperidol [66, 101, 105, 127, 140, 300].
When data are reported in a categorical manner, there is evidence that almost all subjects (72–100 %) receiving oral risperidone [66, 74, 87, 101, 108, 243, 301] and many subjects (53–85 %) receiving risperidone long-acting intramuscular injection have HPRL [66, 172, 243]. The largest randomized controlled trial (n = 555) reporting PRL data found an incidence of 73.8 % (>18 ng/ml in men, >25 ng/ml in women) in risperidone-treated subjects with first-episode psychosis [101]. However, there can be great interindividual differences in the elevation of the serum PRL levels due to risperidone treatment. For example, in one study PRL levels associated with risperidone treatment ranged from 26.9 to 320 ng/ml [142].
At therapeutic concentrations of risperidone, serum PRL levels reach 30–60 ng/ml [240]. However, risperidone can impact on PRL levels even at relatively low doses [115, 140, 302, 303]. Kim et al. [303] found a mean serum PRL concentration with risperidone treatment (mean daily dosage of 3.5 mg/day) of 132.2 ng/ml, after a mean duration of 7.9 weeks. Kinon et al. [140] found that even at doses of risperidone 2 mg/day, PRL levels were already above the upper limit of normal for both females (mean PRL = 76.14 ng/ml) and males (mean PRL = 24.53 ng/ml).
The PRL-elevating effect of risperidone is mostly dose dependent [102, 105, 127, 140, 240, 304–307], even with long-acting risperidone [308]. Turrone et al. [242] showed that risperidone administration results in a dose-related elevation of PRL levels, even after patients have been taking these drugs for a prolonged period of time. Patients receiving risperidone (median dose 3 mg/day, range 1–3 mg/day, baseline plasma PRL level 27 ng/ml, median duration of risperidone treatment 8 months) showed a near doubling over baseline of PRL levels after risperidone administration. Compared with olanzapine and clozapine, whereby PRL levels returned to baseline values by 12–24 h, risperidone-induced PRL levels stayed abnormally high after 24 h.
Although, compared with those on oral risperidone, PRL levels in patients treated with long-acting risperidone seem to be generally lower, even with the long-acting form PRL levels in these patients stay significantly higher than normal PRL values [308–314]. In the phase III study by Chue et al. [309], all patients were first stabilized on oral risperidone, after which they were divided into groups that received long-acting risperidone (25, 50 or 75 mg/day) or were further treated with oral risperidone (2, 4 or 6 mg/day). PRL values for many patients remained elevated in both groups. However, PRL levels dropped significantly (p < 0.001) over the 12-week treatment in the long-acting group, whereas they remained essentially unchanged in the oral group. Mean PRL levels after 12 weeks of treatment were 38 ng/ml for oral risperidone (baseline 38.9 ng/ml) and 32.6 ng/ml for long-acting risperidone (baseline 37.4 ng/ml). This is confirmed in other studies [308, 315]. These observed differences between oral and long-acting risperidone are probably due to the reduced peak-through fluctuations of long-acting risperidone compared with oral risperidone. Nevertheless, long-term administration of oral risperidone seems also to bring about marked decreases in PRL values. Eberhard et al. [178] tracked the course of PRL values over 5 years in psychotic patients treated with risperidone (n = 59). At study entry, the median PRL level of the 218 subjects initially treated with risperidone was 1,522 nmol/l (range 24–5,716 nmol/l) or 66 ng/ml. Although the administration of risperidone was associated with higher PRL values than the values observed with other SGAs during this 5 year period, the investigators noted a strong linear decrease of these values (p < 0.001) with risperidone (year 0: 1,192 nmol/l or 52 ng/ml; year 1: 730 nmol/l or 32 ng/ml; year 2: 533 nmol/l or 23 ng/ml; year 3: 543 nmol/l or 24 ng/ml; year 4: 396 nmol/l or 17 ng/ml; year 5: 412 nmol/l or 18 ng/ml).
Some studies [316, 317] suggest that not risperidone but its major metabolite 9-hydroxyrisperidone (or paliperidone) is the main contributor to the increased serum PRL levels observed in many risperidone-treated patients. Given that risperidone and 9-hydroxyrisperidone have shown similar receptor binding affinities for the D2R, one would expect an almost equally strong D2R blocking effect on the lactotrophs and thus equally elevated PRL levels. However, higher plasma levels of 9-hydroxyrisperidone in treated patients, as well as its longer half-life and lower plasma protein binding, compared with risperidone, has been proposed to explain these results.
6.3.1.8 Sertindole
After it has been suspended in 1998, because of its potential risk in causing cardiovascular-related death, sertindole was relaunched to the European market in 2006. Sertindole has not cause clinically significant PRL elevations (i.e., above normal reference ranges) in short- or long-term clinical studies [299, 318–322]. One study, carried out in 11 European countries, assessing the safety and tolerability of sertindole (modal dose 16 mg/day) in the long-term (18 months) treatment of schizophrenia, found mean serum PRL levels decreased [320]. However, according to a recent meta-analysis, assessing the comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia, sertindole causes statistically significantly higher PRL increases than placebo, which are not statistically significantly less than those induced by haloperidol [299].
6.3.1.9 Ziprasidone
Similar to most SGAs, ziprasidone has a lower propensity for HPRL, compared with FGAs [323]. Most reports indicate a low incidence of PRL elevation and low to moderate levels of HPRL with ziprasidone use [11, 15, 103, 234, 240, 245, 297, 324–326]. Several studies found decreased PRL levels after, respectively, 6 weeks [301], 4–8 weeks [90], 18 weeks [327], 44 weeks [328] and 1 year [329] of treatment with ziprasidone. However, ziprasidone can also be associated with HPRL. In one study, switching from aripiprazole to ziprasidone (mean dose at last observation 116.8 mg/day) in patients with schizophrenia induced a significant increase in serum PRL levels (from 7.2 ng/ml at baseline to 33.8 ng/ml at week 12). HPRL was observed in 54.5 % of the subjects receiving ziprasidone monotherapy [330]. Analysis of the Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE) data showed 17.3 % in the ziprasidone group presented HPRL (men >18.77 ng/ml, women >24.20 ng/ml) [69]. Wu et al. [331] found the increase of serum PRL levels in drug-naïve schizophrenia patients treated with ziprasidone (168 ± 17 mg/day) to be 17 ± 11 and 47 ± 51 μg/l in the ziprasidone male and female group, respectively. Suzuki et al. [332] performed positron emission tomography (PET) scans with [11C]-raclopride in 12 patients with schizophrenia being treated with ziprasidone 60 mg twice daily. PRL levels were highest at 5-h post-dose: PRL levels were higher than the reference range (males 3–13 μg/l; females 3–27 μg/l) in seven patients at 5 h (mean ± standard deviation, SD 23.5 ± 13.6 μg/l). None showed HPRL at 23-h scans. Treatment with ziprasidone can also result in high HPRL rates in certain vulnerable patient groups, such as first-episode patients. Grootens et al. [76], in an 8-week double-blind randomized trial, found that 40 % of patients with recent-onset schizophrenia on ziprasidone (mean study dose 104 mg/day) met criteria for HPRL (>18 ng/ml for men, >25 ng/ml for women) at endpoint. The add-on of ziprasidone to clozapine does not result in significant PRL elevations. Zink et al. [333] found no significant changes in PRL levels in patients who were, after a partial response to monotherapy with clozapine, randomized to the treatment condition in which they received clozapine in combination with ziprasidone (mean dose 134 mg/day) for 6 weeks.
6.3.2 PRL and SGAs in Children and Adolescents
Antipsychotic-induced adverse events can be especially prominent in vulnerable populations such as children and adolescents [46]. With reference to the use of antipsychotic medication in children (≤12 years) and adolescents (12–18 years), one review showed that, in general, all antipsychotic medication, except clozapine, ziprasidone and quetiapine, increase the mean PRL level from baseline values of 8.0 ng/ml to 25–28 ng/ml after 4–8 weeks of treatment (reference range 0–15 ng/ml) [334, 335]. Incidence rates of HPRL during treatment with risperidone, olanzapine and quetiapine in this population were 62, 31 and 12 %, respectively [334]. In the same line, a comprehensive review of prospective head-to-head and placebo-controlled comparisons (34 studies) on the efficacy and safety of SGAs in children and adolescents (n = 2,719) with psychotic and bipolar spectrum disorders found that PRL levels increased the most in subjects on risperidone (mean change ranging from 8.3 to 49.6 ng/ml), followed by olanzapine (−1.5 ng/ml to +13.7 ng/ml). Treatment with aripiprazole was associated with decreased PRL levels, while clozapine and quetiapine were found to be mostly neutral [336]. A recently published systematic review and meta-analysis of the effects of SGAs in children and adolescents aged ≤18 years confirmed these results [337].
6.3.2.1 Aripiprazole
Treatment with aripiprazole in children and adolescents (up to age 18 years) seems, as in adults, to be associated with decreased or unchanged PRL levels [313–318, 336–343]. Moreover, there is also good evidence that PRL levels can decrease even to subnormal PRL serum levels (<3 ng/ml for females and <2 ng/ml for males) [340].
6.3.2.2 Olanzapine
Children and adolescents with schizophrenia may be more susceptible than adults to PRL increases induced by olanzapine [84]. A meta-analysis of double-blind, randomized controlled trials using antipsychotic medications for the treatment of a mental disorder in a pediatric population (children up to 18 years of age) found olanzapine-treated subjects to have much higher odds of an elevated PRL at any time during treatment, compared with placebo (odds ratio 30.52, p < 0.00001) [338]. Another recently published systematic review and meta-analysis confirmed these results [337].
6.3.2.3 Quetiapine
Incidence data of HPRL in children and adolescents treated with quetiapine seem not to be higher than those found for adult patients. A review of 29 studies [334] reported an overall incidence of 12 % during treatment with quetiapine. In the study by Stevens et al. [304], 20 % of the young male adolescents, who had been treated for at least 6 weeks with quetiapine (317.5 ± 238 mg/day), had PRL values that exceeded the normal values (0–15 ng/ml). A comprehensive review of prospective head-to-head and placebo-controlled comparisons (34 studies) on the efficacy and safety of SGAs in children and adolescents (n = 2,719) with psychotic and bipolar spectrum disorders found treatment with quetiapine mostly to be neutral [336].
6.3.2.4 Risperidone
The most and best data concerning children and adolescents are available for risperidone [334]. The meta-analysis of Pringsheim et al. [338] found a change in PRL level from baseline to endpoint to be higher in risperidone-treated versus placebo-treated children, with a mean difference of 44.57 ng/ml (p < 0.00001). Geller et al. [70], in the initial management of children (mean age 11 years) with mania, found risperidone to be associated with HPRL (44.8 ng/ml after 8 weeks of treatment, baseline 7.2 ng/ml, p < 0.001), which raises concern for long-term treatment. Ercan et al. [344] found a significant increase (p < 0.05) in PRL levels after 8 weeks of treatment with risperidone (70 ng/ml at week 8, baseline 5.3 ng/ml) in preschool children (mean age 42.4 months) with conduct disorder and severe behavioral problems. In another study with adolescents and young adults (mean age 16 years) with anorexia nervosa, PRL levels were significantly increased for risperidone-treated subjects at week 7 (54.4 ng/ml, baseline 15.4 ng/ml vs. placebo 8.9 ng/ml, baseline 13.7 ng/ml, p < 0.001) [345]. In a report on a large population of adolescents with schizophrenia (n = 257, mean patient age 15.6 years), Haas et al. [346] observed elevations in PRL to levels >100 ng/ml in 18 % of adolescent schizophrenic patients after 8 weeks of treatment with 4 mg/day of risperidone. Eight short-term risperidone studies (n = 739) with an average duration of 4.6 weeks showed an increase of PRL from 7.9 ng/ml at baseline to 27.6 ng/ml at endpoint. Further treatment showed a decrease to 17.7 ng/ml after 1 year of treatment and to 24.9 ng/ml after 2 years of treatment [334]. In children and adolescents treated with risperidone, this pattern, consistent with the development of PRL tolerance over time, has also been shown by other research. Migliardi et al. [153] found mean PRL levels at 6 and 12 months to be lower than at the first month (baseline 8.4 ng/ml, 1 month 30 ng/ml, 6 months 27.9 ng/ml, 12 months 23.4 ng/ml). Nevertheless, as with olanzapine, vigilance to PRL elevation in all children treated with risperidone is necessary. More rapid CYP2D6 metabolism as well as polymorphic variation in the D2R may be important pharmacogenetic factors determining the risk for risperidone-induced HPRL in children and adolescents [61].
Lawsuits have been filed against Johnson & Johnson alleging that risperidone causes male breast tissue enlargement. The Risperdal FDA warning label states that clinical trials found gynecomastia in 2.3 % of children and adolescents treated with risperidone [347]. Trials have already begun, resulting in some noteworthy settlements [348]. Currently, several law firms are investigating the potential for more side-effect lawsuits regarding gynecomastia in young boys treated with risperidone [348, 349]. Although risperidone, according to a recent evidence-based review, has been identified as one of the medications probably associated with gynecomastia, the authors of this review state that most of the reported drug–gynecomastia associations are based on poor quality evidence [350]. Moreover, gynecomastia has also been mentioned as a potential side effect in other FDA product labels of SGAs (e.g., olanzapine, ziprasidone, quetiapine), FGAs, and selective 5-HT reuptake inhibitors (e.g., sertraline).
6.3.2.5 Ziprasidone
Data on the use of ziprasidone in children are scarce [334, 338]. In general, PRL levels with ziprasidone appear to be lower than what is typically seen in children or adolescents taking olanzapine or risperidone [334, 346]. Moreover, PRL changes with ziprasidone in younger patients mostly are small and transient [351].
6.3.3 PRL and SGAs in First-Episode Patients
Studies have identified younger, antipsychotic-naïve patients with first-episode psychosis as a population vulnerable to cardiovascular and metabolic adverse effects with any antipsychotic [352–354]. As already mentioned above, research has shown that HPRL can occur in about one-third of antipsychotic-naïve first-episode schizophrenic patients, and in about one-fifth of antipsychotic-naïve patients with an at-risk mental state for psychosis [79, 93, 169]. There are indications that this HPRL might be due to a general stress associated with the illness experience and/or to a pre-existing vulnerability [168]. These findings have implications for clinical practice in that PRL should be measured in first-episode patients before starting treatment with antipsychotics [79, 169], not only to ensure that HPRL is not a pre-existing condition, but also, if necessary, to consider choosing a “PRL-sparing” antipsychotic [79]. Furthermore, it might be speculated that stress-induced HPRL plays a role in triggering the outbreak of acute psychotic symptomatology as PRL, through feedback, can increase dopamine. This would make this population even more vulnerable for “PRL-elevating” antipsychotics. However, the latter is certainly speculative and still needs further research [169, 355]. Moreover, the high level of stress, inducing PRL elevations in a substantial proportion of first-episode patients, can be secondary to the acute psychotic symptoms at the moment of admission.
Only a few studies are available that describe PRL changes in first-episode patients treated with SGAs. The results of these studies suggest that the effects of SGAs on PRL-levels in this population follow the same pattern as in adult schizophrenic patients. The presented data do not suggest that, at least for adults, first-episode patients, as a group, are more sensitive to PRL elevations, compared with adult schizophrenic patients. Nevertheless, careful attention should be given to drug selection and the lowest possible effective doses for first-episode patients with HPRL at baseline.
6.3.3.1 Amisulpride
According to the results of the EUFEST study, more first-episode patients on amisulpride (89 %) had HPRL (>18 ng/ml in men and >25 ng/ml in women) than those on the other SGAs [olanzapine (50 %), quetiapine (41 %) and ziprasidone (46 %)] (p = 0.017). Moreover, taking amisulpride resulted in greater increases in PRL values per month (p < 0.0001) [93].
6.3.3.2 Aripiprazole
Aripiprazole seems to be a well-tolerated antipsychotic agent in patients with first-episode schizophrenia. However, few studies have been specifically designed to test the safety of aripiprazole for the treatment of these patients. Lee et al. [211] investigated the therapeutic efficacy and tolerability of aripiprazole for treating first-episode schizophrenia following 8 weeks of treatment in routine clinical conditions. PRL levels after 8 weeks of treatment with aripiprazole were not significantly different from those at baseline. A 26-week prospective study with 300 schizophrenic patients, of whom 106 patients were drug-naïve first-episode patients, found that serum PRL levels significantly decreased from baseline levels (week 8, −20.55 ng/ml, p < 0.001; week 26, −14.46 ng/ml, p < 0.001). Only 10.7 % of patients had an abnormal PRL level (>23 ng/ml) throughout the entire study. There was no significant difference in serum PRL levels between first-episode patients and recurrence patients [83].
6.3.3.3 Olanzapine
Pérez-Iglesias et al. [74] investigated the long-term (up to 1 year) effect of haloperidol, olanzapine and risperidone on serum PRL levels in a naturalistically treated first-episode psychosis population (n = 110). After 1 year, most of the patients in the olanzapine arm had PRL levels that fell within the reference range (<29.2 ng/ml for women and <17.7 ng/ml for men). These findings are in agreement with other studies that have reported no significant or transient PRL elevations with olanzapine treatment [356, 357].
6.3.3.4 Quetiapine
In a small study with only 14 first-episode schizophrenic patients, comparing PRL levels at baseline with those after 12 weeks of treatment with quetiapine, Tauscher-Wisniewski et al. [358] found no significant difference in the elevation of PRL. All PRL levels at baseline and after 12 weeks of quetiapine treatment were in the normal range, with the exception of a slightly elevated level in an antipsychotic-naïve patient at baseline, which resolved at follow-up.
6.3.3.5 Risperidone
A long-term (up to 1 year) study [74], investigating the effect of haloperidol, olanzapine and risperidone on serum PRL levels in a naturalistically treated first-episode psychosis population (n = 110), found that after 1 year of treatment, elevated PRL levels (≥29.2 ng/ml for women and ≥17.7 ng/ml for men) persisted in most patients treated with risperidone. Patients treated with risperidone experienced a substantial increase at 3 months, resulting in PRL levels above the reference range in 90 % of men and 87 % of women. The levels decreased at 1 year, although still more than 70 % of the patients remained above the normative range. Van Bruggen et al. [89] compared the effect of olanzapine and risperidone on hormonal state in 40 patients with a first-episode psychosis (HPRL defined as ≥15 ng/ml for men and ≥22 ng/ml for women). All patients (100 %), both male and female, using risperidone had increased PRL levels, whereas only four patients (17.4 %) (males) using olanzapine had increased PRL levels. Takahashi et al. [357] found that, after an unsuccessful 12-week treatment with olanzapine (mean dose 16.4 mg), plasma PRL concentration significantly increased after switching from olanzapine to risperidone in first-episode patients. Mean PRL concentration of both men and women significantly increased from 6.5 to 16.9 ng/ml and from 17.4 to 54.2 ng/ml, respectively (p < 0.001).
6.3.3.6 Ziprasidone
In an 8-week, open-label, multicenter trial with 27 first-episode patients [359] treated with ziprasidone (mean total daily and endpoint doses were 120.30 ± 40.34 and 131.85 ± 51.22 mg/day, respectively), no significant differences in PRL levels from baseline to last observation were found.
6.4 PRL and Newly Approved Antipsychotics
In 2009 and 2010, three new antipsychotics were approved by the FDA: asenapine (Saphris™, Schering-Plough, Kenilworth, NJ, USA), iloperidone (Fanapt™, Vanda Pharmaceuticals, MD, USA), and lurasidone (Latuda™, Sunovion Pharmaceuticals, Inc., Marlborough, MA, USA) [360]. These newly approved antipsychotics appear to share the same primary mechanism of action with the SGAs. Like most other SGAs, each of these newly approved antipsychotics binds with relatively high potency to 5-HT2A and D2R: iloperidone and asenapine display more potent antagonist activity at 5-HT2AR than D2R, while lurasidone displays relatively equivalent binding at both sites [361, 362]. All three agents are FDA approved for the acute treatment of schizophrenia in adults. Asenapine is also approved for the maintenance treatment of schizophrenia and as a monotherapy or as an adjunct to lithium or valproate for the acute treatment of bipolar manic or mixed episodes, with or without psychotic features [361, 363–370].
6.4.1 Asenapine
Asenapine has a low propensity to cause PRL elevation [361, 371–375]. The PRL profile of asenapine appears to be similar to that of clozapine [376]. Thus, PRL elevation can occur, but at a rate lower than that observed for olanzapine [299, 377, 378] or risperidone [299, 369]. Incidence rates of clinically significant HPRL are generally low [379]. In studies with patients with acute schizophrenia, HPRL (≥2 × upper limit of normal) occurred in 9 % of asenapine recipients (asenapine 5 mg/day) [379, 380]. Incidence rates of marked HPRL (≥4 × upper limit of normal) with asenapine in acute and stable schizophrenia, and bipolar patients were 4–5 % (asenapine 5 and 10 mg BID or twice a day) [376], 2.8 % (asenapine mean dose 17.5 mg/day) [381] and 6.5 % (asenapine mean dose 16.3 mg/day) [382], respectively. Studies [382–385] examining the long-term safety of asenapine in patients with schizophrenia and bipolar disorder showed small mean declines in PRL levels. In the 52-week, double-blind comparison of asenapine (5 or 10 mg BID, n = 913) and olanzapine (10–20 mg once daily, n = 312) by Schoemaker et al. [384], plasma PRL levels decreased within the first 2 weeks from elevated levels at baseline (between 28 and 30 ng/ml) to normal PRL levels in both treatment groups and remained relatively stable during the remainder of the study. During the extension phase [385], there were only minor further changes in PRL levels.
6.4.2 Iloperidone
As with other drugs that antagonize D2R, iloperidone elevates PRL levels [386]. However, the effect of iloperidone on PRL is reported to be low [360, 361, 387–395] and transient with acute onset of treatment [362]. Short-term [389, 396] as well as long-term studies [397] showed PRL levels generally decrease or remain unchanged during iloperidone treatment. However, there does appear to be a potential for PRL elevations in some subjects [389, 398, 399]. In a pooled analysis of three 6-week, prospective, randomized, multicenter, double-blind, placebo- and comparator-controlled trials (n = 1,943), patients were exposed to three dose ranges of iloperidone (4–8, 10–16 and 20–24 mg/day). PRL levels were generally decreased after treatment with two iloperidone dosages; least squares mean changes in PRL from baseline to endpoint were −38 and −23.1 ng/ml in patients receiving iloperidone 4–8 and 10–16 mg/day, respectively. PRL levels were not available for the iloperidone 20–24 mg/day group [396]. However, some other data suggest that higher doses of iloperidone can actually induce HPRL. In a 4-week, double-blind, placebo-controlled trial, 14.8 % of iloperidone (24 mg/day)-treated patients with acute exacerbations of schizophrenia had values outside the upper extended reference range for PRL (not defined), compared with 1.5 % of patients in the placebo group [389]. The insert label of iloperidone, referring to the same trial, however, mentions that elevated plasma PRL levels were observed in 26 % of adults treated with iloperidone 24 mg/day, compared with 12 % in the placebo group [386]. Nevertheless, mean PRL change from baseline remained almost unchanged (+2.6 ng/ml) compared with a decrease of 6.3 ng/ml in the placebo group [389]. Results from a very recent open-label extension trial showed essentially unchanged or decreased levels of serum PRL during long-term (25-week) treatment with iloperidone 24 mg/day (12 mg BID). PRL mean changes from baseline to endpoint for patients switched from ziprasidone and placebo to iloperidone were −7.7 ng/ml (SD 38.7) and +5.1 ng/ml (SD 14.6), respectively. For patients who continued iloperidone treatment, mean PRL change from baseline to endpoint was −15.2 ng/ml (SD 27.9) [397].
6.4.3 Lurasidone
Although lurasidone is reported to induce modest, dose-dependent PRL elevations [400, 401], especially at the beginning of treatment [402], as well as HPRL in some patients [71, 403, 404], in many patients it seems to be associated with no clinically meaningful PRL alterations [71, 405–408].
Product labeling notes that short-term, placebo-controlled trials found small dose- and gender-related (higher in females) effects on PRL levels, with a median change from baseline to endpoint of 0.4 ng/ml for all lurasidone-treated schizophrenic patients (−1.1 ng/ml with 20 mg/day, −1.4 ng/ml with 40 mg/day, −0.2 ng/ml with 80 mg/day, 3.3 ng/ml with 120 and 160 mg/day), compared with −1.9 ng/ml for placebo [404]. The proportion of patients with PRL elevations ≥5 × upper limit of normal (not defined) was 2.8 % for lurasidone-treated patients versus 1 % for placebo-treated patients. For women, this occurred in 5.7 % for lurasidone versus 2 % for placebo; for men in 1.6 % for lurasidone versus 0.6 % for placebo [404]. In a 6-week, double-blind, placebo-controlled trial with a fixed dose of lurasidone 80 mg (n = 90) or placebo (n = 90), treatment with lurasidone was associated with a small but significant median increase in PRL levels at endpoint compared with placebo (+2.4 vs. −0.3 ng/ml, p < 0.05). Larger median increases were observed in the small group of women (+9.2 ng/ml) compared with in men (+1.4 ng/ml). A moderate correlation was seen between serum lurasidone concentrations and PRL levels. Three patients had treatment-emergent PRL concentrations between 50 and 100 ng/ml at endpoint [409]. Pooled data from five double-blind, placebo-controlled, short-term studies of schizophrenia patients (n = 1,004) with an acute exacerbation showed the mean end-point change in PRL (ng/ml) was +1.1 for lurasidone vs. −0.5 for placebo [410].
Consistent with prior short-term studies of lurasidone, there was no indication for clinically relevant changes in PRL in four very recently published short-term studies on lurasidone [401, 403, 411, 412]. In the randomized, 6-week, open-label study of McEvoy et al. [411] (lurasidone 40–120 mg/day), mean changes from baseline to last-observation-carried-forward (LOCF) endpoint in PRL concentrations were −0.5 and 2.0 ng/ml for male and female patients, respectively. PRL ≥5 × upper limit of normal (not defined) was observed in 2/219 subjects (0.9 %), all in the randomized group that initially received lurasidone 80 mg/day for 14 days. Mean change from baseline to LOCF endpoint in PRL concentrations in this dosage group was also higher (9.6 ng/ml) for female patients, compared with all other dosages/gender groups [40 mg/day for 2 weeks and 40 mg/day for 1 week, increased to 80 mg/day on day 8 for week 2 (up-titration group)], where mean changes in PRL concentrations decreased or remained almost unchanged. In the same line, the 6-week, randomized, placebo-controlled study of Nasrallah et al. [403] showed modest increases in PRL following treatment with lurasidone (40, 80 or 120 mg/day), compared with placebo. Median changes from baseline PRL levels were −0.9, 1.3 and 2.4 ng/ml for patients receiving lurasidone 40, 80 and 120 mg/day, respectively, compared with 0.4 ng/ml for those receiving placebo. Changes were greater among female than among male patients, particularly in those receiving lurasidone 120 mg/day (median change, 1.4 ng/ml for men and 6.4 ng/ml for women). However, a change from normal to high (>17.7 ng/ml for men, >29.2 ng/ml for women) PRL levels occurred in 8.3, 16.1 and 19.7 % of patients receiving lurasidone 40, 80 and 120 mg/day, respectively, compared with 4.9 % of patients receiving placebo. Ogasa et al. [401] found median PRL levels at the week 6 LOCF endpoint were modestly increased relative to baseline in the lurasidone 40 (3.5 ng/ml) and 120 mg/day (7.7 ng/ml) groups, but not in the placebo group (−1.3 ng/ml). Again, a sex difference was observed, with lurasidone producing greater increases in PRL in women (3.8 and 21.1 ng/ml in the lurasidone 40 and 120 mg/day, respectively) than in men (3.4 and 5.6 ng/ml in the lurasidone 40 and 120 mg/day, respectively). Two patients discontinued the study because of elevated PRL >200 ng/ml. Finally, Loebel et al. [412], in another 6-week, randomized, double-blind, placebo- and active-controlled trial, found dose-related median changes in PRL levels at the 6-week LOCF endpoint for the lurasidone 80 and 160 mg/day treatment groups to be 0.8 and 3 ng/ml, respectively. This increase in PRL levels was found primarily in female subjects.
Uncontrolled longer-term trials (primarily open-label extensions) reported decreases in PRL concentrations. Lurasidone was associated with a median change in PRL of −0.9 ng/ml at week 24, −5.3 ng/ml at week 36, and −2.2 ng/ml at week 52 [404]. One uncontrolled open-label extension study even reported a mean change of −12.7 ng/ml at week 52 [413]. A very recently published open-label extension study by Stahl et al. [414] observed small changes in PRL levels. PRL mean changes from baseline to endpoint (month 6) for patients switched from olanzapine and placebo to lurasidone were −5.6 ng/ml (SD 10.8) and +6.0 ng/ml (SD 18.9), respectively. For patients who continued lurasidone treatment, mean PRL change from baseline to endpoint was +2.7 ng/ml (SD 37.0). Little change in PRL concentrations from baseline to endpoint were equally reported in controlled trials. A 12-month, double-blind, active-controlled study on the long-term safety and tolerability of lurasidone in schizophrenia showed essentially no change in PRL levels for male as well as female patients treated with once-daily, flexible-dosed lurasidone (40–120 mg). Median changes from baseline to endpoint (month 12) were −0.5 and +0.1 ng/ml for male and female patients, respectively [71].
Recently, a systematic review and exploratory meta-analysis [415] of randomized, placebo-controlled trials was conducted by our research group to assess the efficacy of paliperidone (ER formulation), iloperidone, lurasidone and asenapine on several dimensions of symptoms (positive, negative, general psychopathology, depression, anxiety), as well as their tolerability profile in the short-term treatment (≤12 weeks) of schizophrenia and bipolar disorder, compared with placebo. Although all these antipsychotics statistically significantly (p < 0.05) increased PRL levels more than placebo, paliperidone especially was associated with a higher risk to produce PRL increase, compared with placebo [relative risk 8.52, confidence interval (CI) 4.94–14.67; number needed to harm 2.3, CI 1.3–4.4]. Whereas, compared with placebo, mean changes in PRL levels (ng/ml) with asenapine [weighted mean difference (WMD) 4.517, CI 1.882–7.152] and lurasidone (WMD 9.13, CI 4.9–13.3) treatment were low to moderate, paliperidone was associated with substantial and consistent increases in serum PRL, both in men (WMD 23.85, CI 18.56–29.14) and women (WMD 71.23, CI 54.57–87.89). The mean change in PRL levels (ng/ml) with iloperidone treatment, compared with placebo, was also high (WMD 26.87, CI 12.27–41.47). However, as only one report (containing four trials) on iloperidone was included in this analysis and the heterogeneity value for these results was extremely high (98.5 %), one should consider this result to be unreliable. Therefore, we concluded that asenapine, showing a low propensity to cause PRL elevation at the beginning of treatment, has a PRL profile that appears to be similar to that of clozapine; lurasidone is probably more comparable to ziprasidone and olanzapine; paliperidone elevates PRL levels in a fashion similar to risperidone.
A recently published meta-analysis of 212 blinded, randomized controlled trials (n = 43,049), comparing the efficacy and tolerability of 15 antipsychotic drugs in schizophrenia, found that aripiprazole, quetiapine, asenapine and iloperidone did not cause significantly increased PRL concentrations, compared with placebo. Paliperidone and risperidone were associated with significantly greater PRL increases than all other drugs. Because of methodological reasons, clozapine and amisulpride could not be included in the analysis. The ranking in order of PRL increase presented by these authors is as follows: amisulpride > paliperidone > risperidone > lurasidone > ziprasidone > iloperidone > olanzapine > asenapine > placebo > quetiapine > aripiprazole [299].
Based on our review, PRL side-effect profiles of SGAs and newly approved antipsychotics are given in Table 4.
6.5 Possible Explanations of the PRL-Elevating Tendency of Antipsychotics
Several hypotheses have been put forward to explain the different PRL profiles of antipsychotic medication. A correlation has been proposed between the fastness of dissociation from the D2R and the PRL-sparing properties of certain antipsychotics (“fast dissociation hypothesis”); antipsychotics associated with lesser degrees of PRL elevation have fast D2R dissociation [5]. Clozapine and quetiapine have the fastest rate of dissociation from D2R; olanzapine has an intermediate rate of dissociation [416]. Quetiapine, as an example of an antipsychotic with fast dissociation, is associated with central D2R occupancy that falls from initial blockade of 60–70 % at 2 h post-dosing to around 30 % at 24 h [417]. Conversely, FGAs, as a group, and risperidone dissociate slowly from the D2R, resulting in more prolonged blockade and therefore greater PRL release [5, 418]. However, although its high PRL risk is well known, amisulpride is rapidly dissociated from the D2R [416, 419]. Moreover, according to a recent study, haloperidol, also a highly PRL-releasing antipsychotic, showed a rather fast dissociation profile [420]. It is thus conceivable that other factors, in addition to kinetic properties, contribute to the PRL-releasing properties of antipsychotics.
The different penetration potencies of the various antipsychotics with reference to the blood–brain barrier are another explanation that has been put forward. While the antipsychotic effect is based on the antagonism of receptors situated within the central nervous system, PRL elevation is associated with blockade of D2R at the level of the anterior pituitary lactotroph cells [420]. Anatomically, the pituitary gland lies outside the blood–brain barrier [5, 27]. The lesser ability of amisulpride, sulpiride and risperidone, in comparison with olanzapine and quetiapine, to penetrate this barrier could explain the tendency of these compounds to induce elevated PRL values [80, 316, 418]. Their lesser penetration at therapeutic dose levels leads to a greater D2R occupancy in the pituitary area and therefore to a greater increase of the PRL values [316]. Especially for amisulpride, a decreased penetration of the blood–brain barrier would explain the marked tendency of this medicine to cause a rise in PRL values [195, 421]. This hypothesis is supported by the observation that domperidone, a D2 blocker that does not cross the blood–brain barrier, is associated with significant PRL elevation [422].
The quantification of dopamine D2R occupancy in the pituitary by several antipsychotics using PET scans has not been reported until recently. Arakawa et al. [80] demonstrated that the D2R occupancy in the pituitary was a predictor of HPRL. These researchers measured dopamine D2R binding of risperidone, olanzapine, haloperidol and sulpiride in the pituitary (and temporal) cortex in schizophrenic patients. A significant positive correlation was observed between the plasma concentration of PRL and dopamine D2R occupancy in the pituitary (p = 0.001) by the various antipsychotic drugs. The study also indicated that a 50 % D2R occupancy in the pituitary might represent a threshold level of HPRL. The brain/plasma concentration ratio, indicating the penetrating capability across the blood–brain barrier, seemed to be a good characteristic biomarker of each antipsychotic drug for the risk of HPRL at therapeutic dose.
The largest study to date (n = 481) [69], investigating the relationship between serum PRL concentration and estimated D2R blockade with risperidone (n = 172), olanzapine (n = 211) or ziprasidone (n = 98) in patients with schizophrenia, demonstrated a threshold of HPRL at 73 % of striatal D2R occupancy. However, this threshold seems to differ among antipsychotic drugs. The cut-off points for HPRL were 68–70 % for risperidone, 77 % for olanzapine and 55 % for ziprasidone. This means the threshold for HPRL in D2R occupancy may lie somewhat on the lower side of the established therapeutic window with antipsychotics (i.e., 65–80 %), and therefore highlights the need for the use of the lowest possible dose to avoid this hormonal side effect.
However, D2R blockade on the lactotrophs certainly is not the sole major cause leading to HPRL [49, 420]. In the first systematic examination of the effects of different antidopaminergic mechanisms on PRL concentration in healthy volunteers, Veselinović et al. [49] performed a 7-day intervention with three substances with entirely different mechanisms of action: aripiprazole (partial D2R agonist), haloperidol (D2R antagonist) and reserpine (a vesicular monoamine transporter type 2 blocker). The completely different antidopaminergic mechanisms of haloperidol and reserpine both resulted in a significant increase in PRL levels. PRL levels changed significantly with haloperidol (from 177.2 ± 74.6 to 350.7 ± 202.6 mU/l, p < 0.0001) but not after aripiprazole (from 160.9 ± 65.0 to 189.6 ± 209.6 mU/l, p = 0.69) or placebo (from 211.6 ± 113.4 to 196.1 ± 85.6 mU/l, p = 0.8). However, reserpine, not directly interacting with D2R, caused the most distinct increase (from 149.6 ± 80.2 to 540.3 ± 280.8 mU/l, p < 0.0001). Thus, these findings indicate that the dopaminergic regulation of PRL secretion is not only related to the D2-like dopamine receptors on the lactotroph cells itself. Although PRL release is controlled primarily by dopamine acting on D2R, other neurotransmitter receptor systems probably are involved. Therefore, the serotonergic antagonistic activity of the SGAs has also been proposed as another explanation [423].
Finally, genetic factors also seem to play an important role. Genetic D2R polymorphisms may explain why some individuals are more prone to develop HPRL [424, 425]. The TaqIA A1 [26, 424] and the A-241G alleles are associated with higher PRL concentration, and adverse events potentially related to HPRL were found to be four times more common in TaqIA A1 allele carriers [425].
7 Consequences of HPRL
Multiple signs and symptoms are associated with HPRL (see Table 5) [6, 15, 23, 46, 57, 159, 426, 427].
For years there has been considerable debate about the impact of HPRL in schizophrenic patients. Frequently, HPRL in these patients has been linked to sexual dysfunctions, osteoporosis and even breast cancer.
Although it is often suggested that increased PRL levels are responsible for sexual dysfunctions in schizophrenic patients, one is struck by the fact that the literature on this topic is limited [128, 428] and that the evidence is contradictory and inconclusive [128, 129, 429, 430]. Some studies have shown an association between sexual side effects and PRL elevation, while others have failed to support this [431]. From all evidence taken together, one can conclude that it is highly unlikely that PRL elevation is the sole cause of sexual dysfunctions, and that multiple causes for sexual dysfunction in schizophrenic patients should be considered [5]. Specific pharmacological profiles, especially with regard to the noradrenergic and serotonergic receptors, the impact of the illness itself (e.g., the presence of negative or depressive symptoms), and the impact of patients’ psychosocial settings need to be considered as well [129, 429]. Moreover, a recent study, assessing sexual function in individuals at ultra-high risk of psychosis, showed that sexual function is already impaired prior to the onset of the first episode (and thus initiation of treatment with antipsychotics), with half of these individuals showing evidence of sexual dysfunction. These high rates of dysfunction were not specific to particular domains of sexual function [432].
Also regarding antipsychotic medication and osteoporosis conflicting results exist. Some studies [433–437] suggest antipsychotics contribute to a small increase in the risk of fractures, while others do not [438, 439]. Of the studies examining the relationship between antipsychotic-induced HPRL and bone mineral density loss, about 60 % found some effects of HPRL on bone mineral density [440]. However, as in the case of sexual dysfunctions, the etiology of osteoporosis is multifactorial [441] and the relative contributions of antipsychotic-related HPRL and the influence of important unhealthy lifestyle behaviors in this population (such as smoking and reduced physical activity) remain unclear; more definitive, prospective, long-term studies that directly assess relevant confounding variables are needed [440]. The precise link between HPRL and osteoporosis also remains to be elucidated [5]. There are two potential mechanisms by which HPRL may result in bone mineral density reductions: a direct one in the absence of hypogonadism, and an indirect one mediated (via the hypothalamic–pituitary–gonadal axis) through suppression of gonadal hormone levels [9, 442].
There is accumulating epidemiological, clinical, and biological evidence confirming a role of PRL in human breast cancer risk [8, 443, 444]. However, although an association has been demonstrated, a cause and effect relationship is yet to be established [445]. In vitro and in vivo studies strongly support that PRL is involved in processes related to late-stage carcinogenic effects of breast cancer, including increasing cell proliferation and reducing apoptosis. Thus, it is possible that high circulating PRL levels are important only after a preclinical lesion has developed and promotes late-stage carcinogenesis [443]. The majority of the limited number of studies in which the risk of breast cancer has been investigated in patients treated with FGAs (e.g., [446–448]) or SGAs [449] did not disclose an increased risk of breast cancer. Even exclusive users of PRL-elevating SGAs, such as risperidone, were found not to be at an increased risk of breast cancer [449]. However, based on the above mentioned cancer research on the association between breast cancer risk and PRL, it seems prudent to avoid PRL-elevating medications in women with a history of breast cancer and possibly in those with a strong family history of breast cancer [46, 55, 67, 427].
8 Conclusion
Hyperprolactinemia is often defined as a PRL concentration greater than the upper reference limit of the local laboratory. However, a critical review of the literature shows a substantial variation in reference intervals for serum PRL. Differences in study design, the use of concomitant PRL-elevating medication, the short overall study duration of most studies, the lack of PRL baseline values and information on previous treatments and/or PRL measurements in a substantial number of studies, make the interpretation and comparison of data even more difficult. As rates of HPRL will be dependent on many factors, such as the timing of PRL measurement, these factors may be relevant confounders for some data sets. Units of measurement also cause some confusion: US data are often presented in ng/ml whereas most UK and EU data are in mIU/l; conversion rates are not standardized and vary depending on the assay employed. Finally, clinical reports do not always report the reference interval for serum PRL while speaking about HPRL. Although many studies on PRL are done by pharmaceutical companies, a recent large-scale meta-analysis on antipsychotics (including an analysis on the PRL-inducing properties of these compounds) showed efficacy outcomes did not change substantially when pharmaceutical industry sponsorship was accounted for in meta-regressions and sensitivity analyses [299].
Antipsychotics differ in their propensity to cause PRL elevation. Although a discrimination of “PRL-sparing” versus “PRL-elevating” antipsychotic drugs may provide the clinician with treatment choices in order to avoid or mitigate HPRL, these terms can be misleading for clinicians, concluding that antipsychotic medications such as aripiprazole, clozapine and quetiapine can never be associated with significant HPRL. Actually, all antipsychotics (FGAs, SGAs and newly approved antipsychotics) have the propensity, especially during the first hours of treatment, to elevate PRL levels above the upper limit of normal and to induce HPRL. However, differences between antipsychotic drugs with respect to PRL elevation are large. Among the FGAs, especially the high PRL risk of sulpiride is known. Among the SGAs, amisulpride, risperidone and paliperidone are associated with the greatest elevation of PRL. Olanzapine and ziprasidone do this with a moderate severity. Although clozapine can raise (generally transiently) PRL levels, these mostly remain within normal range. During treatment with quetiapine, PRL levels are mostly found to remain unchanged or to decrease over all dosages, in most cases from levels at switch of treatment. Aripiprazole shows the most benign PRL profile as, in most studies, PRL levels were found to remain unchanged or to decrease, even below those expected from placebo, in adults as well as in children and adolescents. Nevertheless, important consideration must always be given to adolescents and children, who may be more vulnerable than adults to PRL increases and at higher risk of developing HPRL. Recent research also has shown that HPRL can be pre-existing in a substantial proportion of antipsychotic-naïve patients with first-episode psychosis or at-risk mental state. This pre-existing vulnerability and/or a genetic predisposition must therefore be taken into account when considering this important antipsychotic side effect in these populations. Due to the overall paucity of data on the newly approved antipsychotics, there is still insufficient evidence to draw firm conclusions concerning the PRL safety profile of these compounds. Therefore, there is a clear need for further controlled trials to evaluate how safe these newly approved antipsychotics really are and which of these agents are potentially less problematic regarding PRL than most other “older” SGAs.
Prolactin elevations with antipsychotic medication are mostly gender-related and dose-related. However, antipsychotics having a high potential for PRL elevation (amisulpride and risperidone) can have a profound impact on PRL levels even at relatively low doses, while PRL levels with antipsychotics having a minimal effect on PRL (aripiprazole) can remain unchanged over all dosages. Although tolerance and decreases in PRL values after long-term administration of PRL-elevating antipsychotics can occur, these elevations, in most cases, remain above the upper limit of normal.
Notes
Besides monomeric PRL, which accounts for approximately 85 % of total PRL in normal sera, higher-molecular-weight variants of PRL are known. Macroprolactin, which in the majority of cases consists of antigen-antibody complexes of monomeric PRL and immunoglobulin G, contributes <1 % to circulating PRL levels. However, in some patients with supraphysiological PRL levels, macroprolactin was demonstrated to be the predominant form without eliciting the classical signs and symptoms of HPRL [90].
References
Veldhuis JD, Johnson ML. Operating characteristics of the hypothalamo-pituitary-gonadal axis in men: circadian, ultradian, and pulsatile release of prolactin and its temporal coupling with luteinizing hormone. J Clin Endocrinol Metab. 1988;67(1):116–23.
Llovera M, Touraine P, Kelly PA, et al. Involvement of prolactin in breast cancer: redefining the molecular targets. Exp Gerontol. 2000;35(1):41–51.
Nicol M, Willis C, Yiangou C, et al. Relationship between serum prolactin levels and histology of benign and malignant breast lesions: a detailed study of 153 consecutive cases. Breast J. 2002;8(5):281–5.
Fitzgerald P, Dinan TG. Prolactin and dopamine: what is the connection? A review article. J Psychopharmacol. 2008;22(2 Suppl):12–9.
Bushe CJ, Bradley A, Pendlebury J. A review of hyperprolactinaemia and severe mental illness: are there implications for clinical biochemistry? Ann Clin Biochem. 2010;47(Pt 4):292–300.
Cookson J, Hodgson R, Wildgust HJ. Prolactin, hyperprolactinaemia and antipsychotic treatment: a review and lessons for treatment of early psychosis. J Psychopharmacol. 2012;26(5 Suppl):42–51.
Jacobson EM, Hugo ER, Borcherding DC, et al. Prolactin in breast and prostate cancer: molecular and genetic perspectives. Discov Med. 2011;11(59):315–24.
Bernichtein S, Touraine P, Goffin V. New concepts in prolactin biology. J Endocrinol. 2010;206(1):1–11.
Graham SM, Howgate D, Anderson W, et al. Risk of osteoporosis and fracture incidence in patients on antipsychotic medication. Expert Opin Drug Saf. 2011;10(4):575–602.
Hankinson SE, Willett WC, Michaud DS, et al. Plasma prolactin levels and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1999;91(7):629–34.
Hamner M. The effects of atypical antipsychotics on serum prolactin levels. Ann Clin Psychiatry. 2002;14(3):163–73.
Meaney AM, O’Keane V. Prolactin and schizophrenia: clinical consequences of hyperprolactinaemia. Life Sci. 2002;71(9):979–92.
Hummer M, Huber J. Hyperprolactinaemia and antipsychotic therapy in schizophrenia. Curr Med Res Opin. 2004;20(2):189–97.
Goffin V, Touraine P, Culler MD, et al. Drug Insight: prolactin-receptor antagonists, a novel approach to treatment of unresolved systemic and local hyperprolactinemia? Nat Clin Pract Endocrinol Metab. 2006;2(10):571–81.
Petty RG. Prolactin and antipsychotic medications: mechanism of action. Schizophr Res. 1999;35(Suppl):S67–73.
Byerly M, Suppes T, Tran QV, et al. Clinical implications of antipsychotic-induced hyperprolactinemia in patients with schizophrenia spectrum or bipolar spectrum disorders: recent developments and current perspectives. J Clin Psychopharmacol. 2007;27(6):639–61.
La Torre D, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag. 2007;3(5):929–51.
Voicu V, Medvedovici A, Ranetti AE, et al. Drug-induced hypo- and hyperprolactinemia: mechanisms, clinical and therapeutic consequences. Expert Opin Drug Metab Toxicol. 2013;9(8):955–68.
Freeman ME, Kanyicska B, Lerant A, et al. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523–631.
Halbreich U, Kinon BJ, Gilmore JA, et al. Elevated prolactin levels in patients with schizophrenia: mechanisms and related adverse effects. Psychoneuroendocrinology. 2003;28(Suppl 1):53–67.
Emiliano AB, Fudge JL. From galactorrhea to osteopenia: rethinking serotonin-prolactin interactions. Neuropsychopharmacology. 2004;29(5):833–46.
Bergemann N, Parzer P, Mundt C, et al. High bone turnover but normal bone mineral density in women suffering from schizophrenia. Psychol Med. 2008;38(8):1195–201.
Hamner MB, Arana GW. Hyperprolactinaemia in antipsychotic-treated patients. Guidelines for avoidance and management. CNS Drugs. 1998;10(3):209–22.
Radl DB, Zárate S, Jaita G, et al. Apoptosis of lactotrophs induced by D2 receptor activation is estrogen dependent. Neuroendocrinology. 2008;88(1):43–52.
Tuomisto J, Männistö P. Neurotransmitter regulation of anterior pituitary hormones. Pharmacol Rev. 1985;37(3):249–332.
Young RM, Lawford BR, Barnes M, et al. Prolactin levels in antipsychotic treatment of patients with schizophrenia carrying the DRD2*A1 allele. Br J Psychiatry. 2004;185:147–51.
Knegtering R, Castelein S, Bruggeman R. Vaak seksuele functiestoornissen door antipsychotica, vooral bij prolactineverhoging. Resultaten van een aantal vergelijkende onderzoeken. Tijdschrift Voor Seksuologie. 2004;28:140–6.
American Psychiatric Association. Schizophrenia. Am J Psychiatry. 1997;154(Suppl. 4):11–25.
Molitch ME. Medication-induced hyperprolactinemia. Mayo Clin Proc. 2005;80(8):1050–7.
Knegtering H (2003) Antipsychotic treatment and sexual functioning: rol of prolactin. Proefschrift ter verkrijging van het doctoraat in de Medische Wetenschappen aan de Rijksuniversiteit Groningen. http://irs.ub.rug.nl/ppn/254939104.
Coccaro EF, Kavoussi RJ, Oakes M, et al. 5-HT2a/2c receptor blockade by amesergide fully attenuates prolactin response to d-fenfluramine challenge in physically healthy human subjects. Psychopharmacology (Berl). 1996;126(1):24–30.
Xu Y, Jones JE, Lauzon DA, Anderson JG, Balthasar N, Heisler LK, Zinn AR, Lowell BB, Elmquist JK. A serotonin and melanocortin circuit mediates d-fenfluramine anorexia. J Neurosci. 2010;30(44):14630–4.
Murnane KS, Kimmel HL, Rice KC, et al. The neuropharmacology of prolactin secretion elicited by 3,4-methylenedioxymethamphetamine (“ecstasy”): a concurrent microdialysis and plasma analysis study. Horm Behav. 2012;61(2):181–90.
Papageorgiou A, Denef C. Estradiol induces expression of 5-hydroxytryptamine (5-HT) 4, 5-HT5, and 5-HT6 receptor messenger ribonucleic acid in rat anterior pituitary cell aggregates and allows prolactin release via the 5-HT4 receptor. Endocrinology. 2007;148(3):1384–95.
Van de Kar LD, Rittenhouse PA, Li Q, et al. Serotonergic regulation of renin and prolactin secretion. Behav Brain Res. 1996;73(1–2):203–8.
Balsa JA, Sánchez-Franco F, Pazos F, et al. Direct action of serotonin on prolactin, growth hormone, corticotropin and luteinizing hormone release in cocultures of anterior and posterior pituitary lobes: autocrine and/or paracrine action of vasoactive intestinal peptide. Neuroendocrinology. 1998;68(5):326–33.
Van de Kar LD, Javed A, Zhang Y, et al. 5-HT2A receptors stimulate ACTH, corticosterone, oxytocin, renin, and prolactin release and activate hypothalamic CRF and oxytocin-expressing cells. J Neurosci. 2001;21(10):3572–9.
Breton C, Pechoux C, Morel G, et al. Oxytocin receptor messenger ribonucleic acid: characterization, regulation, and cellular localization in the rat pituitary gland. Endocrinology. 1995;136(7):2928–36.
Mogg RJ, Samson WK. Interactions of dopaminergic and peptidergic factors in the control of prolactin release. Endocrinology. 1990;126(2):728–35.
Wanke IE, Rorstad OP. Receptors for vasoactive intestinal peptide in rat anterior pituitary glands: localization of binding to lactotropes. Endocrinology. 1990;126(4):1981–8.
Samson WK, Bianchi R, Mogg RJ, et al. Oxytocin mediates the hypothalamic action of vasoactive intestinal peptide to stimulate prolactin secretion. Endocrinology. 1989;124(2):812–9.
Rittenhouse PA, Levy AD, Li Q, et al. Neurons in the hypothalamic paraventricular nucleus mediate the serotonergic stimulation of prolactin secretion via 5-HT1c/2 receptors. Endocrinology. 1993;133(2):661–7.
Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res. 1996;73(1–2):277–80.
Kiss J, Halász B. Synaptic connections between serotoninergic axon terminals and tyrosine hydroxylase-immunoreactive neurons in the arcuate nucleus of the rat hypothalamus. A combination of electron microscopic autoradiography and immunocytochemistry. Brain Res. 1986;364(2):284–94.
Mirkes SJ, Bethea CL. Oestrogen, progesterone and serotonin converge on GABAergic neurones in the monkey hypothalamus. J Neuroendocrinol. 2001;13(2):182–92.
Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64(20):2291–314.
Chun T-Y, Gregg D, Sarkar DK, et al. Differential regulation by estrogens of growth and prolactin synthesis in pituitary cells suggests that only a small pool of estrogen receptors is required for growth. PNAS. 1998;95(5):2325–30.
Yokoyama Y, Kitchens WC, Toth B, et al. Upregulation of hepatic prolactin receptor gene expression by 17β-estradiol following trauma-hemorrhage. J Appl Physiol. 2003;95(6):2530–6.
Veselinović T, Schorn H, Vernaleken IB, et al. Impact of different antidopaminergic mechanisms on the dopaminergic control of prolactin secretion. J Clin Psychopharmacol. 2011;31(2):214–20.
Messini CI, Dafopoulos K, Chalvatzas N, et al. Effect of ghrelin and thyrotropin-releasing hormone on prolactin secretion in normal women. Horm Metab Res. 2010;42(3):204–8.
Messini CI, Dafopoulos K, Chalvatzas N, et al. Effect of ghrelin and metoclopramide on prolactin secretion in normal women. J Endocrinol Invest. 2011;34(4):276–9.
Benso A, Calvi E, Gramaglia E, Olivetti I, Tomelini M, Ghigo E, Broglio F. Other than growth hormone neuroendocrine actions of ghrelin. Endocr Dev. 2013;25:59–68.
Zhang-Wong JH, Seeman MV. Antipsychotic drugs, menstrual regularity and osteoporosis risk. Arch Womens Ment Health. 2002;5(3):93–8.
Schlechte JA. Clinical practice. Prolactinoma. N Engl J Med. 2003;349(21):2035–41.
Citrome L. Current guidelines and their recommendations for prolactin monitoring in psychosis. J Psychopharmacol. 2008;22(2 Suppl):90–7.
Peuskens J, editor. A literature review of “Prolactin in schizophrenia”. Clear perspectives: management issues in schizophrenia, vol. 1, no. 3 (1997).
Montejo AL. Prolactin awareness: an essential consideration for physical health in schizophrenia. Eur Neuropsychopharmacol. 2008;18(Suppl 2):S108–14.
Brown PJ, Cleghorn JM, Brown GM, et al. Seasonal variations in prolactin levels in schizophrenia. Psychiatry Res. 1988;25(2):157–62.
Garde AH, Hansen AM, Skovgaard LT, et al. Seasonal and biological variation of blood concentrations of total cholesterol, dehydroepiandrosterone sulfate, hemoglobin A(1c), IgA, prolactin, and free testosterone in healthy women. Clin Chem. 2000;46(4):551–9.
Tanner MJ, Hadlow NC, Wardrop R. Variation of female prolactin levels with menopausal status and phase of menstrual cycle. Aust N Z J Obstet Gynaecol. 2011;51(4):321–4.
Rosenbloom AL. Hyperprolactinemia with antipsychotic drugs in children and adolescents. Int J Pediatr Endocrinol. 2010; pii: 159402.
Chahal J, Schlechte J. Hyperprolactinemia. Pituitary. 2008;11(2):141–6.
Mackin P, Waton A, Nulkar A, et al. Prolactin and smoking status in antipsychotic-treated patients. J Psychopharmacol. 2011;25(5):698–703.
Zhang X, Bu R, Sha W, et al. Serum prolactin and smoking status in chronic antipsychotic-treated male patients with schizophrenia. Psychiatry Res. 2013;209(2):239–41.
Ohta C, Yasui-Furukori N, Furukori H, et al. The effect of smoking status on the plasma concentration of prolactin already elevated by risperidone treatment in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):573–6.
Bushe C, Shaw M, Peveler RC. A review of the association between antipsychotic use and hyperprolactinaemia. J Psychopharmacol. 2008;22(2 Suppl):46–55.
Peveler RC, Branford D, Citrome L, et al. Antipsychotics and hyperprolactinaemia: clinical recommendations. J Psychopharmacol. 2008;22(2 Suppl):98–103.
Kelly DL, Wehring HJ, Earl AK, Sullivan K, Dickerson FB, Feldman S, McMahon RP, Buchanan RW, Warfel D, Keller WR, Fischer BA, Shim JC. Treating symptomatic hyperprolactinemia in women with schizophrenia: presentation of the ongoing DAAMSEL clinical trial (Dopamine partial Agonist, Aripiprazole, for the Management of Symptomatic ELevated prolactin). BMC Psychiatry. 2013;13(1):214.
Tsuboi T, Bies RR, Suzuki T, et al. Hyperprolactinemia and estimated dopamine D2 receptor occupancy in patients with schizophrenia: analysis of the CATIE data. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:178–82.
Geller B, Luby JL, Joshi P, et al. A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, children and adolescents. Arch Gen Psychiatry. 2012;69(5):515–28.
Citrome L, Cucchiaro J, Sarma K, et al. Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol. 2012;27(3):165–76.
Kikuchi T, Iwamoto K, Sasada K, et al. Sexual dysfunction and hyperprolactinemia in Japanese schizophrenic patients taking antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):26–32.
Nagai G, Mihara K, Nakamura A, et al. Prolactin concentrations during aripiprazole treatment in relation to sex, plasma drugs concentrations and genetic polymorphisms of dopamine D2 receptor and cytochrome P450 2D6 in Japanese patients with schizophrenia. Psychiatry Clin Neurosci. 2012;66(6):518–24.
Pérez-Iglesias R, Mata I, Martínez-García O, et al. Long-term effect of haloperidol, olanzapine, and risperidone on plasma prolactin levels in patients with first-episode psychosis. J Clin Psychopharmacol. 2012;32(6):804–8.
Sugawara N, Yasui-Furukori N, Fujii A, et al. No association between bone mass and prolactin levels among patients with schizophrenia. Hum Psychopharmacol. 2011;26(8):596–601.
Grootens KP, van Veelen NM, Peuskens J, et al. Ziprasidone vs olanzapine in recent-onset schizophrenia and schizoaffective disorder: results of an 8-week double-blind randomized controlled trial. Schizophr Bull. 2011;37(2):352–61.
Li H, Rui Q, Ning X, et al. A comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1002–8.
Gopal S, Vijapurkar U, Lim P, et al. A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol. 2011;25(5):685–97.
Aston J, Rechsteiner E, Bull N, Borgwardt S, Gschwandtner U, Riecher-Rössler A. Hyperprolactinaemia in early psychosis—not only due to antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1342–4.
Arakawa R, Okumura M, Ito H, et al. Positron emission tomography measurement of dopamine D2 receptor occupancy in the pituitary and cerebral cortex: relation to antipsychotic-induced hyperprolactinemia. J Clin Psychiatry. 2010;71(9):1131–7.
Bushe C, Sniadecki J, Bradley AJ, et al. Comparison of metabolic and prolactin variables from a six-month randomised trial of olanzapine and quetiapine in schizophrenia. J Psychopharmacol. 2010;24(7):1001–9.
Citrome L, Stauffer VL, Chen L, et al. Olanzapine plasma concentrations after treatment with 10, 20, and 40 mg/d in patients with schizophrenia: an analysis of correlations with efficacy, weight gain, and prolactin concentration. J Clin Psychopharmacol. 2009;29(3):278–83.
Kwon JS, Jang JH, Kang DH, et al. Long-term efficacy and safety of aripiprazole in patients with schizophrenia, schizophreniform disorder, or schizoaffective disorder: 26-week prospective study. Psychiatry Clin Neurosci. 2009;63(1):73–81.
Kryzhanovskaya L, Schulz SC, McDougle C, et al. Olanzapine versus placebo in adolescents with schizophrenia: a 6-week, randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry. 2009;48(1):60–70.
Kim SW, Shin IS, Kim JM, et al. Effectiveness of switching to aripiprazole from atypical antipsychotics in patients with schizophrenia. Clin Neuropharmacol. 2009;32(5):243–9.
Byerly MJ, Marcus RN, Tran QV, et al. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. 2009;107(2–3):218–22.
Konarzewska B, Wołczyński S, Szulc A, et al. Effect of risperidone and olanzapine on reproductive hormones, psychopathology and sexual functioning in male patients with schizophrenia. Psychoneuroendocrinology. 2009;34(1):129–39.
Liu-Seifert H, Kinon BJ, Tennant CJ, et al. Sexual dysfunction in patients with schizophrenia treated with conventional antipsychotics or risperidone. Neuropsychiatr Dis Treat. 2009;5:47–54.
van Bruggen M, van Amelsvoort T, Wouters L, et al. Sexual dysfunction and hormonal changes in first episode psychosis patients on olanzapine or risperidone. Psychoneuroendocrinology. 2009;34(7):989–95.
Tschoner A, Engl J, Rettenbacher MA, et al. Is second-generation antipsychotic-induced hyperprolactinemia due to biologically active prolactin or to biologically inactive macroprolactin? Results from a prospective study. J Clin Psychiatry. 2009;70(2):293–4.
Meltzer HY, Bobo WV, Nuamah IF, et al. Efficacy and tolerability of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 6-week, placebo-controlled studies. J Clin Psychiatry. 2008;69(5):817–29.
Emsley R, Berwaerts J, Eerdekens M, et al. Efficacy and safety of oral paliperidone extended-release tablets in the treatment of acute schizophrenia: pooled data from three 52-week open-label studies. Int Clin Psychopharmacol. 2008;23(6):343–56.
Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085–97.
Lu ML, Shen WW, Chen CH. Time course of the changes in antipsychotic-induced hyperprolactinemia following the switch to aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1978–81.
Hanssens L, L’Italien G, Loze JY, et al. The effect of antipsychotic medication on sexual function and serum prolactin levels in community-treated schizophrenic patients: results from the Schizophrenia Trial of Aripiprazole (STAR) study (NCT00237913). BMC Psychiatry. 2008;8:95.
Yuan HN, Wang CY, Sze CW, et al. A randomized, crossover comparison of herbal medicine and bromocriptine against risperidone-induced hyperprolactinemia in patients with schizophrenia. J Clin Psychopharmacol. 2008;28(3):264–370.
Kishimoto T, Watanabe K, Shimada N, et al. Antipsychotic-induced hyperprolactinemia inhibits the hypothalamo-pituitary-gonadal axis and reduces bone mineral density in male patients with schizophrenia. J Clin Psychiatry. 2008;69(3):385–91.
Howes OD, Wheeler MJ, Pilowsky LS, et al. Sexual function and gonadal hormones in patients taking antipsychotic treatment for schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2007;68(3):361–7.
Kinon BJ, Ahl J, Liu-Seifert H, et al. Improvement in hyperprolactinemia and reproductive comorbidities in patients with schizophrenia switched from conventional antipsychotics or risperidone to olanzapine. Psychoneuroendocrinology. 2006;31(5):577–88.
Kelly DL, Conley RR. A randomized double-blind 12-week study of quetiapine, risperidone or fluphenazine on sexual functioning in people with schizophrenia. Psychoneuroendocrinology. 2006;31(3):340–6.
Schooler N, Rabinowitz J, Davidson M, et al. Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry. 2005;162(5):947–53.
Volavka J, Czobor P, Cooper TB, et al. Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychiatry. 2004;65(1):57–61.
Addington DE, Pantelis C, Dineen M, et al. Efficacy and tolerability of ziprasidone versus risperidone in patients with acute exacerbation of schizophrenia or schizoaffective disorder: an 8-week, double-blind, multicenter trial. J Clin Psychiatry. 2004;65(12):1624–33.
Bobes J, Timdahl K. Quetiapine: placebo-level prolactin and low levels of sexual dysfunction. Poster presented at the 17th European College of Neuropsychopharmacology, Stockholm; 2004.
Montgomery J, Winterbottom E, Jessani M, et al. Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry. 2004;65(11):1491–8.
Cavallaro R, Cocchi F, Angelone SM, et al. Cabergoline treatment of risperidone-induced hyperprolactinemia: a pilot study. J Clin Psychiatry. 2004;65(2):187–90.
Kinon BJ, Ahl J, Liu-Seifert H, et al. Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology. 2003;28(Suppl 2):55–68.
Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2003;60(7):681–90.
Canuso CM, Goldstein JM, Wojcik J, et al. Antipsychotic medication, prolactin elevation, and ovarian function in women with schizophrenia and schizoaffective disorder. Psychiatry Res. 2002;111(1):11–20.
Aizenberg D, Modai I, Landa A, et al. Comparison of sexual dysfunction in male schizophrenic patients maintained on treatment with classic antipsychotics versus clozapine. J Clin Psychiatry. 2001;62(7):541–4.
Huber TJ, Rollnik J, Wilhelms J, et al. Estradiol levels in psychotic disorders. Psychoneuroendocrinology. 2001;26(1):27–35.
David SR, Taylor CC, Kinon BJ, et al. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clin Ther. 2000;22(9):1085–96.
Peuskens J, Link CG. A comparison of quetiapine and chlorpromazine in the treatment of schizophrenia. Acta Psychiatr Scand. 1997;96(4):265–73.
Crawford AM, Beasley CM Jr, Tollefson GD. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrations. Schizophr Res. 1997;26(1):41–54.
Walters J, Jones I. Clinical questions and uncertainty—prolactin measurement in patients with schizophrenia and bipolar disorder. J Psychopharmacol. 2008;22(2 Suppl):82–9.
Ali S, Miller KK, Freudenreich O. Management of psychosis associated with a prolactinoma: case report and review of the literature. Psychosomatics. 2010;51(5):370–6.
Alkabbani AG, Mon SY, Hatipoglu B, Kennedy L, Faiman C, Weil RJ, Hamrahian AH. Is a stable or decreasing prolactin level in a patient with prolactinoma a surrogate marker for lack of tumor growth? Pituitary. 2013;. doi:10.1007/s11102-013-0473-5.
Bayrak A, Saadat P, Mor E, Chong L, Paulson RJ, Sokol RZ. Pituitary imaging is indicated for the evaluation of hyperprolactinemia. Fertil Steril. 2005;84(1):181–5.
http://www.pituitary.org/disorders/prolactinomas.aspx. Accessed 17 Sep 2013.
Arcari GT, Mendes AK, Sothern RB. A risperidone-induced prolactinoma resolved when a woman with schizoaffective disorder switched to ziprasidone: a case report. Innov Clin Neurosci. 2012;9(9):21–4.
Broekhof R, Gosselink MJ, Pijl H, et al. The effect of aripiprazole and quinagolide, a dopamine agonist, in a patient with symptomatic pituitary prolactinoma and chronic psychosis. Gen Hosp Psychiatry. 2012;34(2):209.e1–3.
Szarfman A, Tonning JM, Levine JG, et al. Atypical antipsychotics and pituitary tumors: a pharmacovigilance study. Pharmacotherapy. 2006;26(6):748–58.
Lee BJ, Lee SJ, Kim MK, et al. Effect of aripiprazole on cognitive function and hyperprolactinemia in patients with schizophrenia treated with risperidone. Clin Psychopharmacol Neurosci. 2013;11(2):60–6.
Coppola D, Thiagarajah S, Qiu H, et al. Regarding “A risperidone-induced prolactinoma resolved when a woman with schizoaffective disorder switched to ziprasidone: a case report”. Innov Clin Neurosci. 2013;10(2):12–6.
Gianfrancesco FD, Pandina G, Mahmoud R, et al. Potential bias in testing for hyperprolactinemia and pituitary tumors in risperidone-treated patients: a claims-based study. Ann Gen Psychiatry. 2009;8:5.
Serri O, Chik CL, Ur E, et al. Diagnosis and management of hyperprolactinemia. CMAJ. 2003;169(6):575–81.
Kleinberg DL, Davis JM, De Coster R, et al. Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol. 1999;19(1):57–61.
Knegtering H, van den Bosch R, Castelein S, et al. Are sexual side effects of prolactin-raising antipsychotics reducible to serum prolactin? Psychoneuroendocrinology. 2008;33(6):711–7.
Malik P, Kemmler G, Hummer M, EUFEST Study Group, et al. Sexual dysfunction in first-episode schizophrenia patients: results from European First Episode Schizophrenia Trial. J Clin Psychopharmacol. 2011;31(3):274–80.
Biller BM. Diagnostic evaluation of hyperprolactinemia. J Reprod Med. 1999;44(12 Suppl):1095–9.
Bushe C, Yeomans D, Floyd T, et al. Categorical prevalence and severity of hyperprolactinaemia in two UK cohorts of patients with severe mental illness during treatment with antipsychotics. J Psychopharmacol. 2008;22(2 Suppl):56–62.
Yasui-Furukori N, Saito M, Nakagami T, et al. Gender-specific prolactin response to antipsychotic treatments with risperidone and olanzapine and its relationship to drug concentrations in patients with acutely exacerbated schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(3):537–40.
Yasui-Furukori N, Furukori H, Sugawara N, et al. Prolactin fluctuation over the course of a day during treatments with three atypical antipsychotics in schizophrenic patients. Hum Psychopharmacol. 2010;25(3):236–42.
Yasui-Furukori N, Saito M, Tsuchimine S, et al. Association between dopamine-related polymorphisms and plasma concentrations of prolactin during risperidone treatment in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1491–5.
Yasui-Furukori N, Tsuchimine S, Saito M, et al. Association between major Multidrug Resistance 1 (MDR1) gene polymorphisms and plasma concentration of prolactin during risperidone treatment in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1230–4.
Sawamura K, Suzuki Y, Fukui N, et al. Gender differences in prolactin elevation induced by olanzapine in Japanese drug-naïve schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1511–4.
Wode-Helgodt B, Eneroth P, Fyrö B, et al. Effect of chlorpromazine treatment on prolactin levels in cerebrospinal fluid and plasma of psychotic patients. Acta Psychiatr Scand. 1977;56(4):280–93.
Kuruvilla A, Peedicayil J, Srikrishna G, et al. A study of serum prolactin levels in schizophrenia: comparison of males and females. Clin Exp Pharmacol Physiol. 1992;19(9):603–6.
Gründer G, Wetzel H, Schlösser R, et al. Neuroendocrine response to antipsychotics: effects of drug type and gender. Biol Psychiatry. 1999;45(1):89–97.
Kinon BJ, Gilmore JA, Liu H, et al. Hyperprolactinemia in response to antipsychotic drugs: characterization across comparative clinical trials. Psychoneuroendocrinology. 2003;28(Suppl 2):69–82.
Smith S, Wheeler MJ, Murray R, et al. The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol. 2002;22(2):109–14.
Lee BH, Kim YK. The relationship between prolactin response and clinical efficacy of risperidone in acute psychotic inpatients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(4):658–62.
Halbreich U, Kahn LS. Hyperprolactinemia and schizophrenia: mechanisms and clinical aspects. J Psychiatr Pract. 2003;9(5):344–53.
Wudarsky M, Nicolson R, Hamburger SD, et al. Elevated prolactin in pediatric patients on typical and atypical antipsychotics. J Child Adolesc Psychopharmacol. 1999;9(4):239–45.
Frazier JA, Meyer MC, Biederman J, et al. Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry. 1999;38(8):960–5.
Sallee FR, Kurlan R, Goetz CG, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry. 2000;39(3):292–9.
Jordan MP. Ziprasidone-associated galactorrhea in a female teenager. J Am Acad Child Adolesc Psychiatry. 2003;42(1):4–5.
Pappagallo M, Silva R. The effect of atypical antipsychotic agents on prolactin levels in children and adolescents. J Child Adolesc Psychopharmacol. 2004;14(3):359–71.
Hellings JA, Zarcone JR, Valdovinos MG, et al. Risperidone-induced prolactin elevation in a prospective study of children, adolescents, and adults with mental retardation and pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2005;15(6):885–92.
Holzer L, Eap CB. Risperidone-induced symptomatic hyperprolactinaemia in adolescents. J Clin Psychopharmacol. 2006;26(2):167–71.
Madhusoodanan S, Moise D. Risperidone-induced hyperprolactinemia in adolescents: A case series. J Clin Psychiatry. 2006;67(7):1110–3.
Handen BL, Hardan AY. Open-label, prospective trial of olanzapine in adolescents with subaverage intelligence and disruptive behavioral disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(8):928–35.
Migliardi G, Spina E, D’Arrigo C, et al. Short- and long-term effects on prolactin of risperidone and olanzapine treatments in children and adolescents. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1496–501.
Swadi HS, Craig BJ, Pirwani NZ, et al. A trial of quetiapine compared with risperidone in the treatment of first onset psychosis among 15- to 18-year-old adolescents. Int Clin Psychopharmacol. 2010;25(1):1–6.
Masi G, Liboni F. Management of schizophrenia in children and adolescents: focus on pharmacotherapy. Drugs. 2011;71(2):179–208.
Mancini T, Casanueva FF, Giustina A. Hyperprolactinemia and prolactinomas. Endocrinol Metab Clin North Am. 2008;37(1):67–99 vii.
Schyve PM, Smithline F, Meltzer HY. Neuroleptic-induced prolactin level elevation and breast cancer: an emerging clinical issue. Arch Gen Psychiatry. 1978;35(11):1291–301.
Bronstein MD. Disorders of prolactin secretion and prolactinomas. In: DeGroot LL, Jameson LJ, editors. Endocrinology, vol. 1. USA: Elsevier Saunders; 2006.
Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics—a review. Hum Psychopharmacol. 2010;25(4):281–97.
Naidoo U, Goff DC, Klibanski A. Hyperprolactinemia and bone mineral density: the potential impact of antipsychotic agents. Psychoneuroendocrinology. 2003;28(Suppl 2):97–108.
Segal M, Avital A, Rojas M, et al. Serum prolactin levels in unmedicated first-episode and recurrent schizophrenia patients: a possible marker for the disease’s subtypes. Psychiatry Res. 2004;127(3):227–35.
Mazure CM, Quinlan DM, Bowers MB Jr. Recent life stressors and biological markers in newly admitted psychotic patients. Biol Psychiatry. 1997;41(8):865–70.
Rao ML, Gross G, Strebel B, et al. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry. 1994;35(3):151–63.
Keks NA, Copolov DL, Singh BS. Abnormal prolactin response to haloperidol challenge in men with schizophrenia. Am J Psychiatry. 1987;144(10):1335–7.
Meltzer HY, Sachar EJ, Frantz AG. Serum prolactin levels in unmedicated schizophrenic patients. Arch Gen Psychiatry. 1974;31(4):564–9.
Tsigkaropoulou E, Peppa M, Zompola C, et al. Hypogonadism due to hyperprolactinemia and subsequent first episode of psychosis. Gend Med. 2012;9(1):56–60.
Segal M, Avital A, Berstein S, et al. Prolactin and estradiol serum levels in unmedicated male paranoid schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):378–82.
Garcia-Rizo C, Fernandez-Egea E, Oliveira C, et al. Prolactin concentrations in newly diagnosed, antipsychotic-naïve patients with nonaffective psychosis. Schizophr Res. 2012;134(1):16–9.
Riecher-Rössler A, Rybakowski JK, Pflueger MO, et al. Hyperprolactinemia in antipsychotic-naive patients with first-episode psychosis. Psychol Med. 2013;43(12):2571–82.
Rybakowski JK, Dmitrzak-Weglarz M, Kapelski P, et al. Functional −1149g/t polymorphism of the prolactin gene in schizophrenia. Neuropsychobiology. 2012;65(1):41–4.
Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(Suppl 1):4–8.
Doknic M, Maric NP, Britvic D, et al. Bone remodeling, bone mass and weight gain in patients with stabilized schizophrenia in real-life conditions treated with long-acting injectable risperidone. Neuroendocrinology. 2011;94(3):246–54.
Melkersson K, Berinder K, Hulting AL. Effect of antipsychotic-induced hyperprolactinemia on anthropometric measures, insulin sensitivity and lipid profile in patients with schizophrenia or related psychoses. Neuro Endocrinol Lett. 2011;32(4):428–36.
Haefliger T, Bonsack C. Atypical antipsychotics and sexual dysfunction: five case-reports associated with risperidone. Encephale. 2006;32(1 Pt 1):97–105.
Dossenbach M, Hodge A, Anders M, et al. Prevalence of sexual dysfunction in patients with schizophrenia: international variation and underestimation. Int J Neuropsychopharmacol. 2005;8(2):195–201.
Toren P, Ratner S, Laor N, et al. Benefit-risk assessment of atypical antipsychotics in the treatment of schizophrenia and comorbid disorders in children and adolescents. Drug Saf. 2004;27(14):1135–56.
Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res. 1999;35(Suppl):S75–86.
Eberhard J, Lindstrom E, Holstad M, et al. Prolactin level during 5 years of risperidone treatment in patients with psychotic disorders. Acta Psychiatr Scand. 2007;115(4):268–76.
O’Keane V. Antipsychotic-induced hyperprolactinaemia, hypogonadism and osteoporosis in the treatment of schizophrenia. J Psychopharmacol. 2008;22(2 Suppl):70–5.
Marken PA, Haykal RF, Fisher JN. Management of psychotropic-induced hyperprolactinemia. Clin Pharm. 1992;11(10):851–6.
Brown WA, Laughren TP. Tolerance to the prolactin-elevating effect of neuroleptics. Psychiatry Res. 1981;5(3):317–22.
Meltzer HY, Fang VS. The effect of neuroleptics on serum prolactin in schizophrenic patients. Arch Gen Psychiatry. 1976;33(3):279–86.
Meltzer HY, Fang VS. Serum prolactin levels in schizophrenia—effect of antipsychotic drugs: a preliminary report. In: Sachar EJ, editor. Hormones, behaviour, and psychopathology. New York: Raven Press; 1976. p. 177–90.
Igarashi Y, Higuchi T, Toyoshima R, et al. Tolerance to prolactin secretion in the long-term treatment with neuroleptics in schizophrenia. Adv Biochem Psychopharmacol. 1985;40:95–8.
Meltzer HY. Long-term effects of neuroleptic drugs on the neuroendocrine system. Adv Biochem Psychopharmacol. 1985;40:59–68.
Spitzer M, Sajjad R, Benjamin F. Pattern of development of hyperprolactinemia after initiation of haloperidol therapy. Obstet Gynecol. 1998;91(5 Pt 1):693–5.
Suzuki Y, Sugai T, Fukui N, et al. Differences in plasma prolactin levels in patients with schizophrenia treated on monotherapy with five second-generation antipsychotics. Schizophr Res. 2013;145(1–3):116–9.
Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull. 1989;25(3):390–2.
Hagen C, Pedersen PB, Jensen SB, et al. The effect of sulpiride induced hyperprolactinaemia on glucose tolerance and insulin secretion in normal subjects. Clin Endocrinol (Oxf). 1979;10(1):55–60.
Juruena MF, de Sena EP, de Oliveira IR. Safety and tolerability of antipsychotics: focus on amisulpride. Drug Healthc Patient Saf. 2010;2:205–11.
Lee BH, Kang SG, Kim TW, et al. Hyperprolactinemia induced by low-dosage amisulpride in Korean psychiatric patients. Psychiatry Clin Neurosci. 2012;66(1):69–73.
Kopecek M, Bares M, Svarc J, et al. Hyperprolactinemia after low dose of amisulpride. Neuro Endocrinol Lett. 2004;25(6):419–22.
Kopecek M, Bares M, Horacek J. Normalization of hyperprolactinaemia after withdrawal of a low dose of amisulpride. Neuro Endocrinol Lett. 2005;26(4):320.
Holt RI, Peveler RC. Antipsychotics and hyperprolactinaemia: mechanisms, consequences and management. Clin Endocrinol (Oxf). 2011;74(2):141–7.
Andrade C. Low-dose amisulpride and elevation in serum prolactin. J Clin Psychiatry. 2013;74(6):e558–60.
Fric M, Laux G. Prolactin levels and symptoms of hyperprolactinemia in patients treated with amisulpride, risperidone, olanzapine and quetiapine. Psychiatr Prax. 2003;30(Suppl 2):97–101.
McKeage K, Plosker GL. Amisulpride: a review of its use in the management of schizophrenia. CNS Drugs. 2004;18(13):933–56.
Paparrigopoulos T, Liappas J, Tzavellas E, et al. Amisulpride-induced hyperprolactinemia is reversible following discontinuation. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):92–6.
Ahn YM, Lee KY, Kim CE, et al. The acute and long-term effectiveness of amisulpride in patients with schizophrenia: results of a 12-month open-label prospective follow-up study. Hum Psychopharmacol. 2011;26(8):568–77.
Kim EY, Kim SH, Lee NY, et al. Relationship between prolactin levels and subjective endocrine-related adverse effects in patients with schizophrenia receiving long-term treatment with amisulpride. Pharmacopsychiatry. 2012;45(2):57–63.
Frighi V, Stephenson MT, Morovat A, et al. Safety of antipsychotics in people with intellectual disability. Br J Psychiatry. 2011;199(4):289–95.
Swainston Harrison T, Perry CM. Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 2004;64(15):1715–36.
DeLeon A, Patel NC, Crismon ML. Aripiprazole: a comprehensive review of its pharmacology, clinical efficacy, and tolerability. Clin Ther. 2004;26(5):649–66.
Naber D, Lambert M. Aripiprazole: a new atypical antipsychotic with a different pharmacological mechanism. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(8):1213–9.
Fleischhacker WW, McQuade RD, Marcus RN, et al. A double-blind, randomized comparative study of aripiprazole and olanzapine in patients with schizophrenia. Biol Psychiatry. 2009;65(6):510–7.
McIntyre RS, Yoon J, Jerrell JM, et al. Aripiprazole for the maintenance treatment of bipolar disorder: a review of available evidence. Neuropsychiatr Dis Treat. 2011;7:319–23.
Belgamwar RB, El-Sayeh HG. Aripiprazole versus placebo for schizophrenia. Cochrane Database Syst Rev. 2011;(8):CD006622.
Chen CY, Lin TY, Wang CC, et al. Improvement of serum prolactin and sexual function after switching to aripiprazole from risperidone in schizophrenia: a case series. Psychiatry Clin Neurosci. 2011;65(1):95–7.
Park MH, Han C, Pae CU, et al. Aripiprazole treatment for patients with schizophrenia: from acute treatment to maintenance treatment. Expert Rev Neurother. 2011;11(11):1541–52.
Takeuchi H, Uchida H, Suzuki T, et al. Changes in metabolic parameters following a switch to aripiprazole in Japanese patients with schizophrenia: one-year follow-up study. Psychiatry Clin Neurosci. 2010;64(1):104–6.
Lee HY, Ham BJ, Kang RH, et al. Trial of aripiprazole in the treatment of first-episode schizophrenia. Psychiatry Clin Neurosci. 2010;64(1):38–43.
Glick ID, Mankoski R, Eudicone JM, et al. The efficacy, safety, and tolerability of aripiprazole for the treatment of schizoaffective disorder: results from a pooled analysis of a sub-population of subjects from two randomized, double-blind, placebo-controlled, pivotal trials. J Affect Disord. 2009;115(1–2):18–26.
Mir A, Shivakumar K, Williamson RJ, et al. Change in sexual dysfunction with aripiprazole: a switching or add-on study. J Psychopharmacol. 2008;22(3):244–53.
Kane JM, Meltzer HY, Carson WH Jr, et al. Aripiprazole for treatment-resistant schizophrenia: results of a multicenter, randomized, double-blind, comparison study versus perphenazine. J Clin Psychiatry. 2007;68(2):213–23.
Chrzanowski WK, Marcus RN, Torbeyns A, et al. Effectiveness of long-term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52-week, open-label comparison with olanzapine. Psychopharmacology (Berl). 2006;189(2):259–66.
Lee BH. KimYK, Park SH. Using aripiprazole to resolve antipsychotic-induced symptomatic hyperprolactinemia: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(4):714–7.
McQuade RD, Stock E, Marcus R, et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double-blind study. J Clin Psychiatry. 2004;65(Suppl 18):47–56.
Casey DE, Carson WH, Saha AR, Aripiprazole Study Group, et al. Switching patients to aripiprazole from other antipsychotic agents: a multicenter randomized study. Psychopharmacology (Berl). 2003;166(4):391–9.
Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res. 2003;61(2–3):123–36.
Pigott TA, Carson WH, Saha AR, et al. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry. 2003;64(9):1048–56.
Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–71.
Kasper S, Lerman MN, McQuade RD, et al. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol. 2003;6(4):325–37.
Kerwin R, Millet B, Herman E, et al. A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study. Eur Psychiatry. 2007;22(7):433–43.
Byerly MJ, Pikalov A, Marcus RN, et al. Effects of aripiprazole on prolactin levels in patients with schizophrenia during cross titration with risperidone or olanzapine. Poster presented at the 160th annual meeting of the American Psychiatric Association, San Diego; 2007.
Yasui-Furukori N, Furukori H, Sugawara N, et al. Dose-dependent effects of adjunctive treatment with aripiprazole on hyperprolactinemia induced by risperidone in female patients with schizophrenia. J Clin Psychopharmacol. 2010;30(5):596–9.
Chen CK, Huang YS, Ree SC, et al. Differential add-on effects of aripiprazole in resolving hyperprolactinemia induced by risperidone in comparison to benzamide antipsychotics. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(8):1495–9.
Kane JM, Correll CU, Goff DC, et al. A multicenter, randomized, double-blind, placebo-controlled, 16-week study of adjunctive aripiprazole for schizophrenia or schizoaffective disorder inadequately treated with quetiapine or risperidone monotherapy. J Clin Psychiatry. 2009;70(10):1348–57.
Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am J Psychiatry. 2007;164(9):1404–10.
Findling RL, Auby P, Mallikaarjun S, et al. Tolerability of aripiprazole in the treatment of adolescents with schizophrenia. Poster presented at the 160th annual meeting of the American Psychiatric Association, San Diego; 2007.
Wahl R, Ostroff R. Reversal of symptomatic hyperprolactinemia by aripiprazole. Am J Psychiatry. 2005;162(8):1542–3.
Ziadi Trives MZ, Llácer JM, Escudero MA, et al. Effect of the addition of aripiprazole on hyperprolactinemia associated with risperidone long-acting injection. J Clin Psychopharmacol. 2013;33(4):538–41.
Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS ONE. 2013;8(8):e70179.
Paulzen M, Gründer G. Amisulpride-induced hyperprolactinaemia is not reversed by addition of aripiprazole. Int J Neuropsychopharmacol. 2007;10(1):149–51.
Kelly DL, Conley RR. Sexuality and schizophrenia: a review. Schizophr Bull. 2004;30(4):767–79.
Newman-Tancredi A, Kleven MS. Comparative pharmacology of antipsychotics possessing combined dopamine D2 and serotonin 5-HT1A receptor properties. Psychopharmacology (Berl). 2011;216(4):451–73.
Asenjo Lobos C, Komossa K, Rummel-Kluge C, et al. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2010;(11):CD006633.
Markianos M, Hatzimanolis J, Lykouras L. Neuroendocrine responsivities of the pituitary dopamine system in male schizophrenic patients during treatment with clozapine, olanzapine, risperidone, sulpiride, or haloperidol. Eur Arch Psychiatry Clin Neurosci. 2001;251(3):141–6.
de Leon J, Diaz FJ, Josiassen RC, et al. Possible individual and gender differences in the small increases in plasma prolactin levels seen during clozapine treatment. Eur Arch Psychiatry Clin Neurosci. 2004;254(5):318–25.
Melkersson K. Differences in prolactin elevation and related symptoms of atypical antipsychotics in schizophrenic patients. J Clin Psychiatry. 2005;66(6):761–7.
Baggaley M. Sexual dysfunction in schizophrenia: focus on recent evidence. Hum Psychopharmacol. 2008;23(3):201–9.
Breier AF, Malhotra AK, Su TP, et al. Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. Am J Psychiatry. 1999;156(2):294–8.
Turrone P, Kapur S, Seeman MV, et al. Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry. 2002;159(1):133–5.
Bushe C, Shaw M. Prevalence of hyperprolactinaemia in a naturalistic cohort of schizophrenia and bipolar outpatients during treatment with typical and atypical antipsychotics. J Psychopharmacol. 2007;21(7):768–73.
Edwards JG, Barnes TRE. The side-effects of antipsychotic drugs. II. Effects on other physiological systems. In: Barnes T, editor. Antipsychotic drugs and their side-effects. London: Academic Press, Harcourt Brace & Company, Publishers; 1993.
Conley RR, Kelly DL. Second-generation antipsychotics for schizophrenia: a review of clinical pharmacology and medication-associated side effects. Isr J Psychiatry Relat Sci. 2005;42(1):51–60.
Tran PV, Hamilton SH, Kuntz AJ, et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol. 1997;17(5):407–18.
Smith RC, Lindenmayer JP, Davis JM, Kelly E, Viviano TF, Cornwell J, Hu Q, Khan A, Vaidhyanathaswamy S. Effects of olanzapine and risperidone on glucose metabolism and insulin sensitivity in chronic schizophrenic patients with long-term antipsychotic treatment: a randomized 5-month study. J Clin Psychiatry. 2009;70(11):1501–13.
Chen YL, Cheng TS, Lung FW. Prolactin levels in olanzapine treatment correlate with positive symptoms of schizophrenia: results from an open-label, flexible-dose study. Prim Care Companion J Clin Psychiatry. 2009;11(1):16–20.
Kinon BJ, Basson BR, Gilmore JA, et al. Strategies for switching from conventional antipsychotic drugs or risperidone to olanzapine. J Clin Psychiatry. 2000;61(11):833–40.
Karagianis JL, Baksh A. High-dose olanzapine and prolactin levels. J Clin Psychiatry. 2003;64(10):1192–4.
Beasley CM Jr, Tollefson G, Tran P, et al. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology. 1996;14(2):111–23.
Beasley CM Jr, Hamilton SH, Crawford AM, et al. Olanzapine versus haloperidol: acute phase results of the international double-blind olanzapine trial. Eur Neuropsychopharmacol. 1997;7(2):125–37.
Suzuki Y, Ono S, Sugai T, et al. Dose-dependent effects of olanzapine on QT intervals and plasma prolactin levels in Japanese patients with stable schizophrenia. Hum Psychopharmacol. 2011;26(6):440–3.
Hill AL, Sun B, Karagianis JL, et al. Dose-associated changes in safety and efficacy parameters observed in a 24-week maintenance trial of olanzapine long-acting injection in patients with schizophrenia. BMC Psychiatry. 2011;11:28.
Cabaleiro T, López-Rodríguez R, Ochoa D, et al. Polymorphisms influencing olanzapine metabolism and adverse effects in healthy subjects. Hum Psychopharmacol. 2013;28(3):205–14.
López-Rodríguez R, Román M, Novalbos J, et al. DRD2 Taq1A polymorphism modulates prolactin secretion induced by atypical antipsychotics in healthy volunteers. J Clin Psychopharmacol. 2011;31(5):555–62.
Chwieduk CM, Keating GM. Paliperidone extended release: a review of its use in the management of schizophrenia. Drugs. 2010;70(10):1295–317.
Carvalho MM, Góis C. Hyperprolactinemia in mentally ill patients. Acta Med Port. 2011;24(6):1005–12.
Bellantuono C, Santone G. Efficacy, tolerability and safety of paliperidone extended-release in the treatment of schizophrenia and schizoaffective disorder. Riv Psichiatr. 2012;47(1):5–20.
Janicak PG, Winans EA. Paliperidone ER: a review of the clinical trial data. Neuropsychiatr Dis Treat. 2007;3(6):869–97.
Hu S, Yao M, Peterson BS, et al. A randomized, 12-week study of the effects of extended-release paliperidone (paliperidone ER) and olanzapine on metabolic profile, weight, insulin resistance, and β-cell function in schizophrenic patients. Psychopharmacology (Berl). 2013;230(1):3–13.
Kim SW, Chung YC, Lee YH, et al. Paliperidone ER versus risperidone for neurocognitive function in patients with schizophrenia: a randomized, open-label, controlled trial. Int Clin Psychopharmacol. 2012;27(5):267–74.
Montalvo I, Ortega L, López X, et al. Changes in prolactin levels and sexual function in young psychotic patients after switching from long-acting injectable risperidone to paliperidone palmitate. Int Clin Psychopharmacol. 2013;28(1):46–9.
Suzuki H, Gen K, Otomo M, et al. Study of the efficacy and safety of switching from risperidone to paliperidone in elderly patients with schizophrenia. Psychiatry Clin Neurosci. 2013;67(2):76–82.
Einarson TR, Hemels ME, Nuamah I, et al. An analysis of potentially prolactin-related adverse events and abnormal prolactin values in randomized clinical trials with paliperidone palmitate. Ann Pharmacother. 2012;46(10):1322–30.
Sliwa JK, Bossie CA, Fu DJ, et al. Long-term tolerability of once-monthly injectable paliperidone palmitate in subjects with recently diagnosed schizophrenia. Neuropsychiatr Dis Treat. 2012;8:375–85.
Berwaerts J, Xu H, Nuamah I, et al. Evaluation of the efficacy and safety of paliperidone extended-release in the treatment of acute mania: a randomized, double-blind, dose-response study. J Affect Disord. 2012;136(1–2):e51–60.
Fleischhacker WW, Gopal S, Lane R, et al. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neuropsychopharmacol. 2011;22:1–12.
Pandina GJ, Lindenmayer JP, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30(3):235–44.
Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218–26.
Bishara D. Once-monthly paliperidone injection for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2010;6:561–72.
Gopal S, Hough DW, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol. 2010;25(5):247–56.
Canuso CM, Dirks B, Carothers J, et al. Randomized, double-blind, placebo-controlled study of paliperidone extended-release and quetiapine in inpatients with recently exacerbated schizophrenia. Am J Psychiatry. 2009;166(6):691–701.
Canuso CM, Schooler N, Carothers J, et al. Paliperidone extended-release in schizoaffective disorder: a randomized, controlled study comparing a flexible dose with placebo in patients treated with and without antidepressants and/or mood stabilizers. J Clin Psychopharmacol. 2010;30(5):487–95.
Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31–43.
Kane J, Canas F, Kramer M, et al. Treatment of schizophrenia with paliperidone extended-release tablets: a 6-week placebo-controlled trial. Schizophr Res. 2007;90(1–3):147–61.
Davidson M, Emsley R, Kramer M, et al. Efficacy, safety and early response of paliperidone extended-release tablets (paliperidone ER): results of a 6-week, randomized, placebo-controlled study. Schizophr Res. 2007;93(1–3):117–30.
Marder SR, Kramer M, Ford L, et al. Efficacy and safety of paliperidone extended-release tablets: results of a 6-week, randomized, placebo-controlled study. Biol Psychiatry. 2007;62(12):1363–70.
Hough D, Lindenmayer JP, Gopal S, et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(6):1022–31.
Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2–3):107–17.
Kramer M, Litman R, Hough D, et al. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol. 2010;13(5):635–47.
Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology. 2010;35(10):2072–82.
Berwaerts J, Cleton A, Rossenu S, et al. A comparison of serum prolactin concentrations after administration of paliperidone extended-release and risperidone tablets in patients with schizophrenia. J Psychopharmacol. 2010;24(7):1011–8.
Atmaca M, Kuloglu M, Tezcan E. A new atypical antipsychotic: quetiapine-induced sexual dysfunctions. Int J Impot Res. 2005;17(2):201–3.
Arvanitis LA, Miller BG. Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. The Seroquel Trial 13 Study Group. Biol Psychiatry. 1997;42(4):233–46.
Asmal L, Flegar SJ, Wang J, et al. Quetiapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2013;(11):CD006625.
Riedel M, Müller N, Strassnig M, et al. Quetiapine has equivalent efficacy and superior tolerability to risperidone in the treatment of schizophrenia with predominantly negative symptoms. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):432–7.
Buckley PF, Goldstein JM, Emsley RA. Efficacy and tolerability of quetiapine in poorly responsive, chronic schizophrenia. Schizophr Res. 2004;66(2–3):143–50.
Byerly MJ, Lescouflair E, Weber MT, et al. An open-label trial of quetiapine for antipsychotic-induced sexual dysfunction. J Sex Marital Ther. 2004;30(5):325–32.
Small JG, Hirsch SR, Arvanitis LA, et al. Quetiapine in patients with schizophrenia. A high- and low-dose double-blind comparison with placebo. Seroquel Study Group. Arch Gen Psychiatry. 1997;54(6):549–57.
Borison RL, Arvanitis LA, Miller BG. ICI 204,636, an atypical antipsychotic: efficacy and safety in a multicenter, placebo-controlled trial in patients with schizophrenia. U.S. SEROQUEL Study Group. J Clin Psychopharmacol. 1996;16(2):158–69.
Emsley R, Turner HJ, Schronen J, et al. A single-blind, randomized trial comparing quetiapine and haloperidol in the treatment of tardive dyskinesia. J Clin Psychiatry. 2004;65(5):696–701.
Copolov DL, Link CGG, Kowalcyk B. A multicentre, double-blind, randomized comparison of quetiapine (ICI 204,636, ‘Seroquel’) and haloperidol in schizophrenia. Psychol Med. 2000;30(1):95–105.
King DJ, Link CG, Kowalcyk B. A comparison of bd and tid dose regimens of quetiapine (Seroquel) in the treatment of schizophrenia. Psychopharmacology (Berl). 1998;137(2):139–46.
Kapur S, Zipursky R, Jones C, et al. A positron emission tomography study of quetiapine in schizophrenia: a preliminary finding of an antipsychotic effect with only transiently high dopamine D2 receptor occupancy. Arch Gen Psychiatry. 2000;57(6):553–9.
Gaebel W, Schreiner A, Bergmans P, et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial. Neuropsychopharmacology. 2010;35(12):2367–77.
Muench J, Hamer AM. Adverse effects of antipsychotic medications. Am Fam Physician. 2010;81(5):617–22.
Komossa K, Rummel-Kluge C, Schwarz S, et al. Risperidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2011;(1):CD006626.
Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–62.
Yen YC, Lung FW, Chong MY. Adverse effects of risperidone and haloperidol treatment in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(2):285–90.
Zhang H, Li H, Shu L, et al. Double-blind comparison of ziprasidone and risperidone in the treatment of Chinese patients with acute exacerbation of schizophrenia. Neuropsychiatr Dis Treat. 2011;7:77–85.
Knegtering H, Boks M, Blijd C, et al. A randomized open-label comparison of the impact of olanzapine versus risperidone on sexual functioning. J Sex Marital Ther. 2006;32(4):315–26.
Kim KS, Pae CU, Chae JH, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J Clin Psychiatry. 2002;63(5):408–13.
Stevens JR, Kymissis PI, Baker AJ. Elevated prolactin levels in male youths treated with risperidone and quetiapine. J Child Adolesc Psychopharmacol. 2005;15(6):893–900.
Peuskens J. Risperidone in the treatment of patients with chronic schizophrenia: a multi-national, multi-centre, double-blind, parallel-group study versus haloperidol. Risperidone Study Group. Br J Psychiatry. 1995;166(6):712–26.
Compton MT, Miller AH. Antipsychotic-induced hyperprolactinemia and sexual dysfunction. Psychopharmacol Bull. 2002;36(1):143–64.
Haas M, Unis AS, Armenteros J, et al. A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol. 2009;19(6):611–21.
Canuso CM, Bossie CA, Lasser RA, et al. Reduced serum prolactin levels after treatmetn with long-acting risperidone. Poster at 156th APA congress, San Francisco; 2003.
Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol. 2005;15(1):111–7.
Peng PW, Huang MC, Tsai CJ, et al. The disparity of pharmacokinetics and prolactin study for risperidone long-acting injection. J Clin Psychopharmacol. 2008;28(6):726–7.
Bai YM, Chen TT, Lin WK, et al. Pharmacokinetics study for hyperprolactinemia among schizophrenics switched from risperidone to risperidone long-acting injection. J Clin Psychopharmacol. 2007;27(3):306–8.
Verma S, Subramaniam M, Abdin E, et al. Safety and efficacy of long-acting injectable risperidone in patients with schizophrenia spectrum disorders: a 6-month open-label trial in Asian patients. Hum Psychopharmacol. 2010;25(3):230–5.
Bobo WV, Shelton RC. Risperidone long-acting injectable (Risperdal Consta®) for maintenance treatment in patients with bipolar disorder. Expert Rev Neurother. 2010;10(11):1637–58.
Louzã MR, Elkis H, Ruschel S, et al. Long-acting injectable risperidone in partially adherent and nonadherent patients with schizophrenia. Neuropsychiatr Dis Treat. 2011;7:391–8.
Szerman N, Basurte-Villamor I, Montes JM, et al.. Prolactin levels and side effects of long-acting risperidone injection and oral treatments in psychotic disorders. Poster at 20th ECNP congress, Vienna; 2007.
Knegtering R, Baselmans P, Castelein S, et al. Predominant role of the 9-hydroxy metabolite of risperidone in elevating blood prolactin levels. Am J Psychiatry. 2005;162(5):1010–2.
Melkersson KI. Prolactin elevation of the antipsychotic risperidone is predominantly related to its 9-hydroxy metabolite. Hum Psychopharmacol. 2006;21(8):529–32.
Muscatello MR, Bruno A, Pandolfo G, et al. Emerging treatments in the management of schizophrenia - focus on sertindole. Drug Des Dev Ther. 2010;4:187–201.
van Kammen DP, McEvoy JP, Targum SD, et al. A randomized, controlled, dose ranging trial of sertindole in patients with schizophrenia. Psychopharmacology (Berl). 1996;124(1–2):168–75.
Hale AS, Azorin JM, Lemming OM, et al. Sertindole in the long-term treatment of schizophrenia. Int Clin Psychopharmacol. 2012;27(4):231–7.
Murdoch D, Keating GM. Sertindole: a review of its use in schizophrenia. CNS Drugs. 2006;20(3):233–55.
Spina E, Zoccali R. Sertindole: pharmacological and clinical profile and role in the treatment of schizophrenia. Expert Opin Drug Metab Toxicol. 2008;4(5):629–38.
Citrome L. Drug safety evaluation of ziprasidone. Expert Opin Drug Saf. 2011;10(3):437–48.
Lusskin SI, Cancro R, Chuang L, et al. Prolactin elevation with ziprasidone. Am J Psychiatry. 2004;161(10):1925.
Anghelescu I, Wolf J. Successful switch to aripiprazole after induction of hyperprolactinemia by ziprasidone: a case report. J Clin Psychiatry. 2004;65(9):1286–7.
Stip E, Zhornitsky S, Moteshafi H, et al. Ziprasidone for psychotic disorders: a meta-analysis and systematic review of the relationship between pharmacokinetics, pharmacodynamics, and clinical profile. Clin Ther. 2011;33(12):1853–67.
Sacchetti E, Galluzzo A, Valsecchi P, et al. Ziprasidone vs clozapine in schizophrenia patients refractory to multiple antipsychotic treatments: the MOZART study. Schizophr Res. 2009;110(1–3):80–9.
Addington DE, Labelle A, Kulkarni J, et al. A comparison of ziprasidone and risperidone in the long-term treatment of schizophrenia: a 44-week, double-blind, continuation study. Can J Psychiatry. 2009;54(1):46–54.
Arato M, O’Connor R, Meltzer HY, et al. A 1-year, double-blind, placebo-controlled trial of ziprasidone 40, 80 and 160 mg/day in chronic schizophrenia: the Ziprasidone Extended Use in Schizophrenia (ZEUS) study. Int Clin Psychopharmacol. 2002;17(5):207–15.
Kim SW, Shin IS, Kim JM, et al. Effectiveness of switching from aripiprazole to ziprasidone in patients with schizophrenia. Clin Neuropharmacol. 2010;33(3):121–5.
Wu XL, Wang JH, Hu SH, et al. Serum prolactin levels and the acute-phase efficacy in drug-naïve schizophrenia treated with ziprasidone and olanzapine (translated version). East Asian Arch Psychiatry. 2012;22(1):7–11.
Suzuki T, Graff-Guerrero A, Uchida H, et al. Dopamine D2/3 occupancy of ziprasidone across a day: a within-subject PET study. Psychopharmacology (Berl). 2013;228(1):43–51.
Zink M, Kuwilsky A, Krumm B, et al. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomised controlled clinical trial. J Psychopharmacol. 2009;23(3):305–14.
Roke Y, van Harten PN, Boot AM, et al. Antipsychotic medication in children and adolescents: a descriptive review of the effects on prolactin level and associated side effects. J Child Adolesc Psychopharmacol. 2009;19(4):403–14.
Correll C, Kishimoto T, Carlson H. Review: most antipsychotic drugs more than double the prolactin levels in children and adolescents. Evid Based Ment Health. 2010;13(2):54.
Fraguas D, Correll CU, Merchán-Naranjo J, et al. Efficacy and safety of second-generation antipsychotics in children and adolescents with psychotic and bipolar spectrum disorders: comprehensive review of prospective head-to-head and placebo-controlled comparisons. Eur Neuropsychopharmacol. 2011;21(8):621–6.
Almandil NB, Liu Y, Murray ML, et al. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15(2):139–50.
Pringsheim T, Lam D, Ching H, et al. Metabolic and neurological complications of second-generation antipsychotic use in children: a systematic review and meta-analysis of randomized controlled trials. Drug Saf. 2011;34(8):651–68.
Doey T. Aripiprazole in pediatric psychosis and bipolar disorder: a clinical review. J Affect Disord. 2012;138(Suppl):S15–21.
Safer DJ, Calarge CA, Safer AM. Prolactin serum concentrations during aripiprazole treatment in youth. J Child Adolesc Psychopharmacol. 2013;23(4):282–9.
Yoo HK, Joung YS, Lee JS, et al. A multicenter, randomized, double-blind, placebo-controlled study of aripiprazole in children and adolescents with Tourette’s disorder. J Clin Psychiatry. 2013;74(8):e772–80.
Datta SS, Kumar A, Wright SD, et al. Evidence base for using atypical antipsychotics for psychosis in adolescents. Schizophr Bull. 2013;. doi:10.1093/schbul/sbt196.
Kumar A, Datta SS, Wright SD,et al. Atypical antipsychotics for psychosis in adolescents. Cochrane Database Syst Rev 2013;(10):CD009582. doi:10.1002/14651858.CD009582.pub2.
Ercan ES, Basay BK, Basay O, et al. Risperidone in the treatment of conduct disorder in preschool children without intellectual disability. Child Adolesc Psychiatry Ment Health. 2011;5(1):10.
Hagman J, Gralla J, Sigel E, et al. A double-blind, placebo-controlled study of risperidone for the treatment of adolescents and young adults with anorexia nervosa: a pilot study. J Am Acad Child Adolesc Psychiatry. 2011;50(9):915–24.
Haas M, Eerdekens M, Kushner S, et al. Efficacy, safety and tolerability of two dosing regimens in adolescent schizophrenia: double-blind study. Br J Psychiatry. 2009;194(2):158–64.
Highlights of prescribing information. Risperdal. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020272S-055S-058S-061S-062lbl.pdf. Revised Aug 2010. Accessed 14 Jan 2014.
http://injurylawyer-news.com/risperdal/gynecomastia/. Accessed 14 Jan 2014.
http://westrikeback.com/practice-areas/dangerous-defective-drugs/risperdal/. Accessed 14 Jan 2014.
Deepinder F, Braunstein GD. Drug-induced gynecomastia: an evidence-based review. Expert Opin Drug Saf. 2012;11(5):779–95.
Elbe D, Carandang CG. Focus on ziprasidone: a review of its use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2008;17(4):220–9.
De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–26.
Pramyothin P, Khaodhiar L. Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):460–6.
Zhang JP, Gallego JA, Robinson DG, et al. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2013;16(6):1205–18.
Shrivastava A, Johnston M, Bureau Y, et al. Baseline serum prolactin in drug-naive, first-episode schizophrenia and outcome at five years: is it a predictive factor? Innov Clin Neurosci. 2012;9(4):17–21.
Bicikova M, Hampl R, Hill M, et al. Neuro- and immunomodulatory steroids and other biochemical markers in drug-naive schizophrenia patients and the effect of treatment with atypical antipsychotics. Neuro Endocrinol Lett. 2011;32(2):141–7.
Takahashi H, Yoshida K, Ishigooka J, et al. Switching to risperidone after unsuccessful treatment of olanzapine in the first-episode schizophrenia: an open trial. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):1067–72.
Tauscher-Wisniewski S, Kapur S, Tauscher J, et al. Quetiapine: an effective antipsychotic in first-episode schizophrenia despite only transiently high dopamine-2 receptor blockade. J Clin Psychiatry. 2002;63(11):992–7.
Zhao T, Park TW, Yang JC, et al. Efficacy and safety of ziprasidone in the treatment of first-episode psychosis: an 8-week, open-label, multicenter trial. Int Clin Psychopharmacol. 2012;27(4):184–90.
Miyake N, Miyamoto S, Jarskog LF. New serotonin/dopamine antagonists for the treatment of schizophrenia: are we making real progress? Clin Schizophr Relat Psychoses. 2012;6(3):122–33.
Bobo WV. Asenapine, iloperidone and lurasidone: critical appraisal of the most recently approved pharmacotherapies for schizophrenia in adults. Expert Rev Clin Pharmacol. 2013;6(1):61–91.
Weiden PJ. Iloperidone for the treatment of schizophrenia: an updated clinical review. Clin Schizophr Relat Psychoses. 2012;6(1):34–44.
McIntyre RS, Wong R. Asenapine: a synthesis of efficacy data in bipolar mania and schizophrenia. Clin Schizophr Relat Psychoses. 2012;5(4):217–20.
McIntyre RS. Asenapine: a review of acute and extension phase data in bipolar disorder. CNS Neurosci Ther. 2011;17(6):645–8.
Szegedi A, Zhao J, van Willigenburg A, et al. Effects of asenapine on depressive symptoms in patients with bipolar I disorder experiencing acute manic or mixed episodes: a post hoc analysis of two 3-week clinical trials. BMC Psychiatry. 2011;11:101.
Szegedi A, Verweij P, van Duijnhoven W, et al. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73(12):1533–40.
Cazorla P, Mackle M, Zhao J, et al. Safety and tolerability of switching to asenapine from other antipsychotic agents: pooled results from two randomized multicenter trials in stable patients with persistent negative symptoms in schizophrenia. Neuropsychiatr Dis Treat. 2012;8:247–57.
Pompili M, Venturini P, Innamorati M, et al. The role of asenapine in the treatment of manic or mixed states associated with bipolar I disorder. Neuropsychiatr Dis Treat. 2011;7:259–65.
Citrome L. Role of sublingual asenapine in treatment of schizophrenia. Neuropsychiatr Dis Treat. 2011;7:325–39.
Citrome L, Nasrallah HA. On-label on the table: what the package insert informs us about the tolerability profile of oral atypical antipsychotics, and what it does not. Expert Opin Pharmacother. 2012;13(11):1599–613.
Gonzalez JM, Thompson PM, Moore TA. Review of the safety, efficacy, and side effect profile of asenapine in the treatment of bipolar 1 disorder. Patient Prefer Adherence. 2011;5:333–41.
Potkin SG. Asenapine: a clinical overview. J Clin Psychiatry. 2011;72(Suppl 1):14–8.
Chwieduk CM, Scott LJ. Asenapine: a review of its use in the management of mania in adults with bipolar I disorder. CNS Drugs. 2011;25(3):251–67.
McIntyre RS. Pharmacology and efficacy of asenapine for manic and mixed states in adults with bipolar disorder. Expert Rev Neurother. 2010;10(5):645–9.
Bishara D, Taylor D. Asenapine monotherapy in the acute treatment of both schizophrenia and bipolar I disorder. Neuropsychiatr Dis Treat. 2009;5:483–90.
Kane JM, Cohen M, Zhao J, et al. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30(2):106–15.
McIntyre R, Alphs L, Cohen M, et al. Long-term double-blind extension studies of asenapine vs. olanzapine in patients with bipolar mania. Schizophr Res. 2008;98(Suppl 1):48–9.
McIntyre R, Alphs L, Cohen M, et al. Long-term double-blind extension studies of asenapine vs olanzapine in patients with bipolar mania. J Affect Disord. 2008;107(Suppl 1):S91–2.
Weber J, McCormack PL. Asenapine. CNS Drugs. 2009;23(9):781–92.
Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psychiatry. 2007;68(10):1492–500.
Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry. 2011;72(3):349–55.
McIntyre RS, Cohen M, Zhao J, et al. Asenapine for long-term treatment of bipolar disorder: a double-blind 40-week extension study. J Affect Disord. 2010;126(3):358–65.
Emsley R, Doelder PD, Schoemaker J, et al. Long-term safety of asenapine in patients with schizophrenia. Schizophr Res. 2008;98(Suppl 1):48.
Schoemaker J, Naber D, Vrijland P, et al. Long-term assessment of asenapine vs. olanzapine in patients with schizophrenia or schizoaffective disorder. Pharmacopsychiatry. 2010;43(4):138–46.
Schoemaker J, Stet L, Vrijland P, et al. Long-term efficacy and safety of asenapine or olanzapine in patients with schizophrenia or schizoaffective disorder: an extension study. Pharmacopsychiatry. 2012;45(5):196–203.
Fanapt (iloperidone) package insert. Rockville: Vanda Pharmaceuticals; 2013. http://www.pharma.us.novartis.com/product/pi/pdf/fanapt.pdf. Accessed 26 Sep 2013.
Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783–91.
Kelleher JP, Centorrino F, Albert MJ, et al. Advances in atypical antipsychotics for the treatment of schizophrenia: new formulations and new agents. CNS Drugs. 2002;16(4):249–61.
Cutler AJ, Kalali AH, Weiden PJ, et al. Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol. 2008;28(2 Suppl 1):S20–8.
Cutler AJ. Iloperidone: a new option for the treatment of schizophrenia. Expert Rev Neurother. 2009;9(12):1727–41.
Citrome L. Iloperidone: chemistry, pharmacodynamics, pharmacokinetics and metabolism, clinical efficacy, safety and tolerability, regulatory affairs, and an opinion. Expert Opin Drug Metab Toxicol. 2010;6(12):1551–64.
Bishop JR, Bishop DL. Iloperidone for the treatment of schizophrenia. Drugs Today (Barc). 2010;46(8):567–79.
Arif SA, Mitchell MM. Iloperidone: a new drug for the treatment of schizophrenia. Am J Health Syst Pharm. 2011;68(4):301–8.
Citrome L. Iloperidone: a clinical overview. J Clin Psychiatry. 2011;72(Suppl 1):19–23.
Crabtree BL, Montgomery J. Iloperidone for the management of adults with schizophrenia. Clin Ther. 2011;33(3):330–45.
Weiden PJ, Cutler AJ, Polymeropoulos MH, et al. Safety profile of iloperidone: a pooled analysis of 6-week acute-phase pivotal trials. J Clin Psychopharmacol. 2008;28(2 Suppl 1):S12–9.
Cutler AJ, Kalali AH, Mattingly GW, et al. Long-term safety and tolerability of iloperidone: results from a 25-week, open-label extension trial. CNS Spectr. 2013;18(1):43–54.
Albers LJ, Musenga A, Raggi MA. Iloperidone: a new benzisoxazole atypical antipsychotic drug. Is it novel enough to impact the crowded atypical antipsychotic market? Expert Opin Investig Drugs. 2008;17(1):61–75.
Citrome L. Iloperidone redux: a dissection of the Drug Approval Package for this newly commercialised second-generation antipsychotic. Int J Clin Pract. 2010;64(6):707–18.
McIntyre RS, Cha DS, Alsuwaidan M, et al. A review of published evidence reporting on the efficacy and pharmacology of lurasidone. Expert Opin Pharmacother. 2012;13(11):1653–9.
Ogasa M, Kimura T, Nakamura M, et al. Lurasidone in the treatment of schizophrenia: a 6-week, placebo-controlled study. Psychopharmacology (Berl). 2013;225(3):519–30.
Samalin L, Garnier M, Llorca PM. Clinical potential of lurasidone in the management of schizophrenia. Ther Clin Risk Manag. 2011;7:239–50.
Nasrallah HA, Silva R, Phillips D, et al. Lurasidone for the treatment of acutely psychotic patients with schizophrenia: a 6-week, randomized, placebo-controlled study. J Psychiatr Res. 2013;47(5):670–7.
Latuda (prescribing information). Sunovion Pharmaceuticals Inc. http://www.latuda.com/LatudaPrescribingInformation.pdf. Revised Dec 2012, Accessed 18 Sep 2013.
Yasui-Furukori N. Update on the development of lurasidone as a treatment for patients with acute schizophrenia. Drug Des Dev Ther. 2012;6:107–15.
Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2011;65(2):189–210.
Owen RT. Lurasidone: a new treatment option for schizophrenia. Drugs Today (Barc). 2011;47(11):807–16.
Sanford M. Lurasidone: in the treatment of schizophrenia. CNS Drugs. 2013;27(1):67–80.
Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829–36.
Cucchiaro J, Pikalov A, Ogasa M, et al. Safety of lurasidone: pooled analysis of five placebo-controlled trials in patients with schizophrenia. Int J Neuropsychopharmacol. 2010;13(Suppl 1):217.
McEvoy JP, Citrome L, Hernandez D, et al. Effectiveness of lurasidone in patients with schizophrenia or schizoaffective disorder switched from other antipsychotics: a randomized, 6-week, open-label study. J Clin Psychiatry. 2013;74(2):170–9.
Loebel A, Cucchiaro J, Sarma K, et al. Efficacy and safety of lurasidone 80 mg/day and 160 mg/day in the treatment of schizophrenia: a randomized, double-blind, placebo- and active-controlled trial. Schizophr Res. 2013;145(1–3):101–9.
Hiroki O, Masakumi M, Masaaki O, et al. A prospective, 1-year, open-label, flexible dose study of lurasidone in the treatment of schizophrenia: safety, tolerability and effectiveness. Poster presented at the 164th annual meeting American Psychiatric Association, Honolulu; 2011 (Abst NR06-22).
Stahl SM, Cucchiaro J, Simonelli D, et al. Effectiveness of lurasidone for patients with schizophrenia following 6 weeks of acute treatment with lurasidone, olanzapine, or placebo: a 6-month, open-label, extension study. J Clin Psychiatry. 2013;74(5):507–15.
Yu WP, Detraux J, Sweers K, van Winkel R, Correll CU, De Hert M. Effectiveness and safety of asenapine, iloperidone, lurasidone, and paliperidone in the short-term treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis of randomized placebo-controlled trials (unpublished report).
Möller HJ. Amisulpride: limbic specificity and the mechanism of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1101–11.
Langlois X, Megens A, Lavreysen H, et al. Pharmacology of JNJ-37822681, a specific and fast-dissociating D2 antagonist for the treatment of schizophrenia. J Pharmacol Exp Ther. 2012;342(1):91–105.
Kapur S, Langlois X, Vinken P, et al. The differential effects of atypical antipsychotics on prolactin elevation are explained by their differential blood–brain disposition: a pharmacological analysis in rats. J Pharmacol Exp Ther. 2002;302(3):1129–34.
De Bartolomeis A, Marmo F, Buonaguro EF, et al. Imaging brain gene expression profiles by antipsychotics: region-specific action of amisulpride on postsynaptic density transcripts compared to haloperidol. Eur Neuropsychopharmacol. 2013;23(11):1516–29.
Carboni L, Negri M, Michielin F, Bertani S, Fratte SD, Oliosi B, Cavanni P. Slow dissociation of partial agonists from the D2 receptor is linked to reduced prolactin release. Int J Neuropsychopharmacol. 2012;15(5):645–56.
Bressan RA, Erlandsson K, Spencer EP, et al. Prolactinemia is uncoupled from central D2/D3 dopamine receptor occupancy in amisulpride treated patients. Psychopharmacology (Berl). 2004;175(3):367–73.
Kapur S, Zipursky R, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156(2):286–93.
Meltzer H, Massey B. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr Opin Pharmacol. 2011;11(1):59–67.
Calarge CA, Ellingrod VL, Acion L, et al. Variants of the dopamine D2 receptor gene and risperidone-induced hyperprolactinemia in children and adolescents. Pharmacogenet Genomics. 2009;19(5):373–82.
Houston JP, Fijal B, Heinloth AN, et al. Genetic associations of prolactin increase in olanzapine/fluoxetine combination-treated patients. Psychiatry Res. 2010;175(1–2):171–2.
Smith SM. The impact of hyperprolactinaemia on sexual function in patients with psychosis. J. Psychopharmacol. 2008;22(2 Suppl):63–9.
Vyas U. Risk of breast cancer due to hyperprolactinemia caused by antipsychotics (neuroleptics). BJMP. 2012;5(4):a534.
Park YW, Kim Y, Lee JH. Antipsychotic-induced sexual dysfunction and its management. World J Mens Health. 2012;30(3):153–9.
Rettenbacher MA, Hofer A, Ebenbichler C, et al. Prolactin levels and sexual adverse effects in patients with schizophrenia during antipsychotic treatment. J Clin Psychopharmacol. 2010;30(6):711–5.
Westheide J, Cvetanovska G, Albrecht C, et al. Prolactin, subjective well-being and sexual dysfunction: an open label observational study comparing quetiapine with risperidone. J Sex Med. 2008;5(12):2816–26.
De Hert M, Detraux J. Novel and newly approved antipsychotics, serum prolactin levels and sexual dysfunctions: a critical literature review. Expert Opin Drug Saf. 2014 (in press).
Marques TR, Smith S, Bonaccorso S, et al. Sexual dysfunction in people with prodromal or first-episode psychosis. Br J Psychiatry. 2012;201:131–6.
Takkouche B, Montes-Martínez A, Gill SS, et al. Psychotropic medications and the risk of fracture: a meta-analysis. Drug Saf. 2007;30(2):171–84.
Howard L, Kirkwood G, Leese M. Risk of hip fracture in patients with a history of schizophrenia. Br J Psychiatry. 2007;190:129–34.
Hugenholtz GW, Heerdink ER, van Staa TP, et al. Risk of hip/femur fractures in patients using antipsychotics. Bone. 2005;37(6):864–70.
Vestergaard P, Rejnmark L, Mosekilde L. Anxiolytics, sedatives, antidepressants, neuroleptics and the risk of fracture. Osteoporos Int. 2006;17(6):807–16.
Jacqmin-Gadda H, Fourrier A, Commenges D, et al. Risk factors for fractures in the elderly. Epidemiology. 1998;9(4):417–23.
Guo Z, Wills P, Viitanen M, et al. Cognitive impairment, drug use, and the risk of hip fracture in persons over 75 years old: a community-based prospective study. Am J Epidemiol. 1998;148(9):887–92.
Bolton JM, Metge C, Lix L, et al. Fracture risk from psychotropic medications: a population-based analysis. J Clin Psychopharmacol. 2008;28(4):384–91.
Kishimoto T, De Hert M, Carlson HE, et al. Osteoporosis and fracture risk in people with schizophrenia. Curr Opin Psychiatry. 2012;25(5):415–29.
Javaid MK, Holt RI. Understanding osteoporosis. J Psychopharmacol. 2008;22(2 Suppl):38–45.
Crews MP, Howes OD. Is antipsychotic treatment linked to low bone mineral density and osteoporosis? A review of the evidence and the clinical implications. Hum Psychopharmacol. 2012;27(1):15–23.
Tworoger SS, Eliassen AH, Zhang X, Qian J, Sluss PM, Rosner BA, Hankinson SE. A 20-year prospective study of plasma prolactin as a risk marker of breast cancer development. Cancer Res. 2013;73(15):4810–9.
Harvey PW. Hypothesis: prolactin is tumorigenic to human breast: dispelling the myth that prolactin-induced mammary tumors are rodent-specific. J Appl Toxicol. 2012;32(1):1–9.
Sethi BK, Chanukya GV, Nagesh VS. Prolactin and cancer: Has the orphan finally found a home? Indian J Endocrinol Metab. 2012;16(Suppl 2):S195–8.
Wagner S, Mantel N. Breast cancer at a psychiatric hospital before and after the introduction of neuroleptic agents. Cancer Res. 1978;38(9):2703–8.
Kanhouwa S, Gowdy JM, Solomon JD. Phenothiazines and breast cancer. J Natl Med Assoc. 1984;76(8):785–8.
Kelly JP, Rosenberg L, Palmer JR, et al. Risk of breast cancer according to use of antidepressants, phenothiazines, and antihistamines. Am J Epidemiol. 1999;150(8):861–8.
Azoulay L, Yin H, Renoux C, et al. The use of atypical antipsychotics and the risk of breast cancer. Breast Cancer Res Treat. 2011;129(2):541–8.
Conflict of interest
Joseph Peuskens declares that he has been a consultant for, received grant and/or research support and honoraria from, and has been on the speakers’ bureaus and/or advisory boards of the following companies: Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Lundbeck and Pfizer.
Luca Pani declares that he has no conflict of interest.
Marc De Hert declares that he has been a consultant for, received grant and/or research support and honoraria from, and has been on the speakers’ bureaus and/or advisory boards of the following companies: Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck, Pfizer, Sanofi Aventis and Takeda.
Johan Detraux declares that his work for the Belgian Discussion Board on Antipsychotic Treatment, established by Janssen and consisting of Belgian psychiatrists discussing relevant topics on antipsychotic treatment, has been partially supported by the Janssen Academy.
No sources of funding were used to conduct this study and/or to prepare the review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Peuskens, J., Pani, L., Detraux, J. et al. The Effects of Novel and Newly Approved Antipsychotics on Serum Prolactin Levels: A Comprehensive Review. CNS Drugs 28, 421–453 (2014). https://doi.org/10.1007/s40263-014-0157-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0157-3