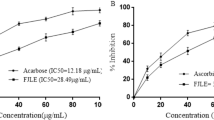
Abstract
Background
Diabetes mellitus (DM) is one of the leading causes of death globally and complications of DM have become a major health concern. Anacardium occidentale is a plant widely recognized for its hypoglycemic properties and traditionally used in developing nations as remedy for DM treatment. Riboceine is a supplement that enhances production of glutathione and known for its vital role in supporting cellular function. This study was designed to evaluate the antidiabetic and antioxidant potential of riboceine and ethanolic extract of A. occidentale leaves in streptozotocin (STZ)-induced diabetic rats.
Method
Twenty-nine adult male Wistar rats were induced with DM intraperitoneally using a single dose of STZ (70 mg/kg). The STZ-induced rats were divided into groups and administered the same dose (100 mg/kg) of A. occidentale leaves extract and riboceine via gastric gavage at the dose (100 mg/kg) for seventeen days while metformin (40 mg/kg) was used as positive control. Fasting blood glucose and weight of the model rats were examined periodically. Activities of total protein, creatinine, urea, antioxidants (SOD, GSH and GPX), and level of serum insulin were determined. Expression of diabetes related genes including pancreas (Insulin, pdx-1, P16NK4A, and Mki-67), Liver (FAS, ACC, and GFAT) and KIM-1 genes were also determined.
Results
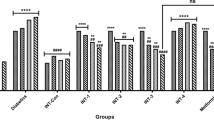
Data showed that treatment of STZ-induced diabetic rats with A. occidentale and riboceine at the same dose significantly (p < 0.05) ameliorated hyperglycemic effects by improving hepatic and renal functions and antioxidants, preventing hepatic fat accumulation by downregulation of ACC, FAS and GFAT expression, improving β-cell functions through up-regulation of pancreatic insulin, P16NK4A, Mki-67 and pdx-1 expression. Induction of diabetes upregulated mRNA expression of KIM-1, which was ameliorated after treatment of the rats with A. occidentale and riboceine.
Conclusion
The results obtained in this study demonstrate significant antidiabetic properties of ethanolic extract of A. occidentale and riboceine.








Similar content being viewed by others
References
Elekofehinti OO, et al. Discovery of potential visfatin activators using in silico docking and ADME predictions as therapy for type 2 diabetes. Beni-suef Univ J Basic Appl Sci. 2018;7:241–249.
Osadebe PO, Odoh EU, Uzor PF. The search for new hypoglycemic agents from plant. Afr J Pharm Pharmacol. 2014;8(11):292–303. https://doi.org/10.5897/AJPP2014.3933.
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Emerging Risk Factors Collaboration. Lancet. 2010;375(9733):2215–2222. https://doi.org/10.1016/S0140-6736(10)60484-9
Jangid H, Chaturvedi S, Khinchi M. An overview on diabetis mellitus. Asian J Pharm Res Dev. 2017;1–11.
Elekofehinti OO, Ayodele OC, Iwaloye O. Momordica charantia nanoparticles promote mitochondria biogenesis in the pancreas of diabetic-induced rats: gene expression study. Egypt J Med Hum Genet. 2015;22:80. https://doi.org/10.1186/s43042-021-00200-w.
Centers for disease control and prevention. What is diabetes? https://www.cdc.gov/diabetes/basics/diabetes.html. Accessed 20 Feb 2022
Wesam K, Maryam F, Zahra A, Damoon A, Majid A. The role of medicinal plants in the treatment of diabetes: a systematic review. 2016;8(1):1832–1842. https://doi.org/10.19082/1832.
Rose K, Haikael DM, Judith K. Traditional Medicine and Its Role in the Management of Diabetes Mellitus: (Patients’ and Herbalists’ Perspectives). Evid Based Complement Alternat Med. 2019. https://doi.org/10.1155/2019/2835691.
Elekofehinti OO, Ariyo EO, Iwaloye O, Obafemi TO. Co-administration of metformin and gallic acid modulates JAK/STAT signaling pathway and glutathione metabolism in fructose-fed streptozotocin diabetic Rats. Phytomedi Plus. 2022;100181. https://doi.org/10.1016/j.phyplu.2021.100181.
Teixeira CC, et al. Absence of anti-hyperglycaemic effect of Jambolan in experimental and clinical models. J Ethnopharmacol. 2000;71:343–7.
Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587–91. https://doi.org/10.2147/DMSO.S67400.
Shoback DG, Gardner D, eds. Greenspan's basic & clinical endocrinology (9th ed.). McGraw-Hill Medical. 2011.
Bindu J, Narendhirakannan RT. Role of medicinal plants in the management of diabetes mellitus: a review. Biotech. 2009;9:4. https://doi.org/10.1007/s13205-018-1528-0.
Dean L, McEntyre J. The genetic landscape of diabetes [Internet]. National Center for Biotechnology Information (US), Bethesda. 2004.
Cooke DW, Plotnick L. Type 1 diabetes mellitus in pediatrics. Pediatr Rev. 2008;29(11):374–84. https://doi.org/10.1542/pir.29-11-374.
Kenny C. When hypoglycemia is not obvious: diagnosing and treating under-recognized and undisclosed hypoglycemia. Prim Care Diabetes. 2014;8(1):3–11. https://doi.org/10.1016/j.pcd.2013.09.002.PMID24100231.
Sarwar N, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–22. https://doi.org/10.1016/S0140-6736(10)60484-9.
O’Gara PT, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):362–425. https://doi.org/10.1161/CIR.0b013e3182742cf6.
Cukierman T. Cognitive decline and dementia in diabetes – systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–9. https://doi.org/10.1007/s00125-005-0023-4.
American Diabetes, Association. Lifestyle Management: Standards of Medical Care in Diabetes. Diabetes Care. 2019;42(1):46–60. https://doi.org/10.2337/dc19-S005.
Rosberger DF. Diabetic retinopathy: current concepts and emerging therapy. Endocrinol Metab Clin North Am. 2013;42(4):721–45. https://doi.org/10.1016/j.ecl.2013.08.001.
Thanabalasingham G, Owen KR. Diagnosis and management of maturity onset diabetes of the young (MODY). BMJ. 2011;343(3):6044. https://doi.org/10.1136/bmj.d6044.
Sattar N, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–42. https://doi.org/10.1016/S0140-6736(09)61965-6.
Kooti W, et al. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: A review. J HerbMed Pharmacol. 2015;4:1–9.
Elekofehinti OO, Adewumi NA, Iwaloye O. Antidiabetic potential of Chromolaena Odorata leave extract and its effect on Nrf2/keap1 antioxidant pathway in the liver of diabetic-induced Wistar Rats. Adv Tradit Med. 2021. https://doi.org/10.1007/s13596-021-00618-y.
Ajiboye BO, Iwaloye O, Owolabi OV, et al. Screening of potential antidiabetic phytochemicals from Gongronema latifolium leaf against therapeutic targets of type 2 diabetes mellitus: multi-targets drug design. SN Appl Sci. 2015;4:14. https://doi.org/10.1007/s42452-021-04880-2.
Afrisham R, et al. Inhibitory Effect of Heracleumpersicum and Ziziphus jujuba on Activity of Alpha-Amylase. J Bot. 2015;1–8. https://doi.org/10.1155/2015/824683.
Gondi M, et al. Anti‐diabetic effect of dietary mango (Mangifera indica L.) peel in streptozotocin‐induced diabetic rats. J Sci Food Agric. 2014;95(5):991–9. https://doi.org/10.1002/jsfa.6778.
Olawale F, Olofinsan K, Iwaloye O. Biological activities of Chromolaena odorata: A mechanistic review. S Afr J Bot. 2022;144:44–57.
Kubo I, Nitoda T, Tocoli FE, Green IR. Multifunctional cytotoxic agents from Anacardium occidentale. Phytother Res. 2011;25(1):38–45.
Kulis M, et al. Pepsinized cashew proteins are hypoallergenic and immunogenic and provide effective immunotherapy in micewith cashew allergy. J Allergy Clin Immunol. 2012;30(3):716–23.
Castillo-Juarez I, Rivero-Cruz F, Celis H, Romero I. Anti-Helicobacter pylori activity of anacardic acids from Amphipterygium adstringens. J Ethnopharmacol. 2007;11401:72–7.
Olajide OA, Aderogba MA, Adedapo ADA, Makinde JM. Effects of Anacardium occidentale stem bark extract on in vivo inflammatory models. J Ethnopharmacol. 2004;95(3):139–42.
Olajide OA, Aderogba MA, Fiebich BL. Mechanisms of anti-inflammatory property of Anacardium occidentale stem bark: Inhibition of NF-κB and MAPK signalling in the microglia. J Ethnopharmacol. 2013;145(1):42–9.
Jaiswal Y, Tatke P, Gabhe SY, Vaidya A. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J Tradit Complement Med. 2016;1:7.
Lawrence AO, John IO, Ayodele OS. Antidiabetic Effect of Anacardium occidentale. Stem-Bark in Fructose-Diabetic Rats. Pharm Biol. 2005;43(7):589–93. https://doi.org/10.1080/13880200500301712.
Palheta IC, Ferreira LR. Hypoglycemic potential of Anacardium occidentale L. J Anal Pharm Res. 2018;7(2):152–1 53. https://doi.org/10.15406/japlr.2018.07.00216.
Ukwenya VO, Adelakun SA, Elekofehinti OO. Exploring the antidiabetic potential of compounds isolated from Anacardium occidentale using computational aproach: ligand-based virtual screening. In Silico Pharmacol. 2021;3(9):25. https://doi.org/10.1007/s40203-021-00084-z.
Ukwenya VO, Ashaolu OJ, Adeyemi DO, Abraham KJ. Experimental diabetes and the epididymis of Wistar rats: The protective effects of Anacardium occidentale (Linn.). J Exp Clin Anat. 2015;14:57–62.
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–5.
Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–90.
Rotruck JT, et al. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–90. https://doi.org/10.1126/science.179.4073.588.
Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–66.
Folorunso IM, Olawale F, Olofinsan K, Iwaloye O. Picralima nitida leaf extract ameliorates oxidative stress and modulates insulin signaling pathway in high fat-diet/STZ induced diabetic rats. S Afr J Bot. 2022;148:268–82.
Sokeng SD, et al. Hypoglycemic Effect of Anacardium occidentale L. Methanol Extract and Fractions on Streptozotocin-induced Diabetic Rats. Glob J Pharmacol. 2007;1(1):01–05.
Saidu AN, Akanya HO, Dauda BEN, Ogbadoyi EO. Antibacterial and comparative hypoglycemic effect of Anacardium occidentale leaves. Int Res J Biochem Bioinformatics. 2012;2(1):006–10.
Dionísio AP, e al. Cashew-apple (Anacardium occidentale L.) and yacon (Smallanthus sonchifolius) functional beverage improve the diabetic state in rats. Food Res Int. 2015;77:171–176.
Jaiswal YS, Tatke PA, Gabhe YS, Vaidya AB. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J Tradit Complement Med Vol. 2017;7(4):421–427.
Obaineh OM, Shadrach A. Phytochemical Constituents and Medicinal Properties of Different Extracts of Anacardium Occidentale and Psidium Guajava. Asian J Biomed Pharm Sci. 2013;3(16):20–3.
Ojezele OA, Agunbiade S. Phytochemical Constituents and Medicinal Properties of Different Extracts of Anacardium Occidentale and Psidium Guajava. Asian J Biomed Pharm Sci. 2013;3(16):2–23.
Awodele O, et al. Toxicological evaluation of therapeutic and supra-therapeutic doses of Cellgevity® on reproductive function and biochemical indices in Wistar rats. BMC Pharmacol Toxicol. 2018;19:68. https://doi.org/10.1186/s40360-018-0253-y.
Rajendran P, et al. Antioxidants and human diseases. Clin Chim Acta. 2014;436:332–47.
Kaleem M, Asif M, Ahmed QU, Bano B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore Med J. 2006;47:670–5.
Searle AJ, Wilson RL. Glutathione peroxidase: Effect of superoxide, hydroxyl and bromine free radicals on enzyme activity. Int J Radiat Biol Relat Stud Phys Chem Med. 1980;37:213.
Freeman BA, Crapo JD. Biology of disease: Free radicals and tissue injury. Lab Invest. 1982;47:412.
Main PA, et al. The potential role of the antioxidant and detoxification properties of glutathione in autism spectrum disorders: a systematic review and meta-analysis. Nutr Metab (Lond). 2012;9:35. https://doi.org/10.1186/1743-7075-9-35.
Jaiswal YS, Tatke PA, Gabhe SY, Ashok V. Antioxidant activity of various extracts of leaves of Anacardium occidentale(cashew). Res J Pharm Biol Chem Sci. 2010;1:112–9.
Chotphruethipong L, Benjakul S, Kijroongrojana K. Optimization of extraction of antioxidative phenolic compounds from cashew (Anacardium occidentale L.) leaves using response surface methodology. J Food Biochem. 2017;41:12379.
Huda-Faujan N, Rahim ZA, Rehan MM, Ahmad FH. Comparative analysis of phenolic content and antioxidative activities of eight Malaysian traditional vegetables. Malay J Anal Sci. 2015;19:611–24.
Kongkachuichai R, et al. Nutrients value and antioxidant content of indigenous vegetables from Southern Thailand. Food Chem. 2015;173:838–46.
Pasupathi P, Chandrasekar V, Senthil Kumar U. Evaluation of oxidative stress, enzymatic and non-enzymatic antioxidants and metabolic thyroid hormone status in patients with diabetes mellitus. Diabetes and metabolic syndrome. Clin Res Rev. 2009;3:160–165.
Chandramohan G, Al-Numair KS, Pugalendi KV. Effect of 3-hydroxymethyl xylitol on hepatic and renal functional markers and protein levels in streptozotocin diabetic rats. Afr J Biochem Res. 2009;3:198–204.
Zulcafli AS, et al. Antidiabetic Potential of Syzygium sp.: An Overview. The Yale journal of biology and medicine. 2020;93(2):307–325.
Ajiboye BO, et al. Ameliorative Activity of Ethanolic Extract of Artocarpus heterophyllus Stem Bark on Alloxan-induced Diabetic Rats. Adv Pharm Bull. 2018;8(1):141–7. https://doi.org/10.15171/apb.2018.017.
AMAAghajanyan A, Movsisyan Z, Trchounian A. Antihyperglycemic and Antihyperlipidemic Activity of Hydroponic Stevia rebaudiana Aqueous Extract in Hyperglycemia Induced by Immobilization Stress in Rabbits. Biomed Res Int. 2017;9251358. https://doi.org/10.1155/2017/9251358.
Moodley K, et al. Antioxidant, antidiabetic and hypolipidemic effects of tulbaghia violacea harv. (wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complement Altern Med. 2015;15:408. https://doi.org/10.1186/s12906-015-0932-9.
Sartang NM, Mazloomi SM, Tanideh N, Zadeh AR. The effects of probiotic soymilk fortified with omega-3 on blood glucose, lipid profile, haematological and oxidative stress, and inflammatory parameters in streptozotocin. J Diabetes Res. 2015. https://doi.org/10.1155/2015/696372
Nukatsuka M, Yoshimura Y, Nishid AM, Kawada J. Importance of the concentration of ATP in rat pancreatic beta cells in the mechanism of streptozotocin-induced cytotoxicity. J Endocrinol. 1990;127:161–5.
Bedoya FJ, Solano F, Lucas M. N-monomethyl-arginine and nicotinamide prevent streptozotocin-induced double strand DNA break formation in pancreatic rat islets. Experientia. 1996;52:344–7.
Sai Varsha MKN, Thiagarajan R, Manikandan R, Dhanasekaran G. Vitamin K1 alleviates streptozotocin-induced type 1 diabetes by mitigating free radical stress, as well as inhibiting NF-kB activation and iNOS expression in rat pancreas. Nutrition. 2015;31:214–22.
Ngubane PS, Hadebe SI, Serumula MR, Musabayane CT. The effects of transdermal insulin treatment of streptozotocin-induced diabetic rats on kidney function and renal expression of glucose transporters. Ren Fail. 2015;37(1):151–9.
Gouda W, et al. Effects of nano-curcumin on gene expression of insulin and insulin receptor. Bull Natl Res Cent. 2019;43:128. https://doi.org/10.1186/s42269-019-0164-0.
Kouhsari SM, Moradabadi L, San MF. Hypoglycemic Effects of Three Medicinal Plants in Experimental Diabetes: Inhibition of Rat Intestinal α-glucosidase and Enhanced Pancreatic Insulin and Cardiac Glut-4 mRNAs Expression. Iran J Pharm Res. 2013;12(3):387–97.
Jayaprasada B, Sharavanana PS, Sivarajb R. Antidiabetic effect of Chloroxylon swietenia bark extracts on streptozotocin induced diabetic rats. Beni-Suef Univ J Basic Appl Sci. 2016;5(1):61–69.
Fujimoto K, Polonsky KS. xPdx1 and other factors that regulate pancreatic β-cell survival Diabetes Obes Metab. 2009;11(4):30–37. https://doi.org/10.1111/j.1463-1326.2009.01121.
Spaeth JM, et al. Defining a Novel Role for the Pdx1 Transcription Factor in Islet β-Cell Maturation and Proliferation During Weaning. Diabetes. 2017;66(11):2830–2839. https://doi.org/10.2337/db16-1516.
Marghani B, Ateya A, Saleh R, Eltaysh R. Antidiabetic and Ameliorative Effect of Lupin Seed Aqueous Extract on Hyperglycemia, Hyperlipidemia and Effect on pdx1, Nkx6.1, Insulin-1, GLUT-2 and Glucokinase Genes Expression in Streptozotocin-induced Diabetic Rats. J Food Nutr Res. 2019;7:333–341.
Whitfield ML, George LK, Grant GD, Perou CM. Common markers of proliferation. Nat Rev Cancer. 2006;6:99–106.
Anne PL, Clay J, Semenkovich F. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta. 2012;1821(5):747–53. https://doi.org/10.1016/j.bbalip.2011.09.017.
Semenkovich CF. Regulation of fatty acid synthase (FAS). Prog Lipid Res. 1997;36(1):43–53.
Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes FASEB J. 1994;8(15):1248–1259.
Paulauskis JD, Sul HS. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J Biol Chem. 1989;264(1):574–7.
Tong L. Acetyl-coenzyme A carboxylase: crucial metabolic enzyme and attractive target for drug discovery. Cell Mol Life Sci. 2005;62(16):1784–803. https://doi.org/10.1007/s00018-005-5121-4.PMID15968460.
Bin-Jumah MN. Monolluma quadrangula protects against oxidative stress and modulates LDL receptor and fatty acid synthase gene expression in hypercholesterolemic rats. Oxid Med Cell Longev. 2018;3914384:10.
Xie Z, et al. Nuciferine prevents hepatic steatosis by regulating lipid metabolismin diabetic rat model. Open Life Sci. 2019;14:699–706.
Kuo-Chen C. Molecular Therapeutic Target for Type-2 Diabetes. Proteome Res. 2004;3(6):1284–8. https://doi.org/10.1021/pr049849v.
Kolm-Litty V, et al. High glucose induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101:160–9.
Du XL, et al. Hyperglycemia induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci. 2000;97:12222–6.
Khan AB, Fatima SS, Khan GM, Shahid S. Evaluation of kidney injury molecule-1 as a disease progression biomarker in diabetic nephropathy. Pak J Med Sci. 2019;35(4):992–996. https://doi.org/10.12669/pjms.35.4.154.
Plotnikov EY, et al. The role of oxidative stress in acute renal injury of newborn rats exposed to hypoxia and endotoxin. FEBS J. 2017;284:3069–78.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ukwenya, V.O., Alese, M.O., Ogunlade, B. et al. Anacardium occidentale leaves extract and riboceine mitigate hyperglycemia through anti-oxidative effects and modulation of some selected genes associated with diabetes. J Diabetes Metab Disord 22, 455–468 (2023). https://doi.org/10.1007/s40200-022-01165-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01165-2