Abstract
The leaf sample from okra plants showing the yellow vein mosaic disease symptoms was collected in Karnataka state, India. The genome of the virus was amplified, cloned and sequenced. Sequence analysis revealed that the viral genome (GU112065) is 2,741 bp in length and genome is similar to that of monopartite begomoviruses originating from the Old World, with seven conserved ORFs. Further nucleotide (nts) sequence comparisons showed that the genome has the highest sequence identities of 96.1 % with Bhendi yellow vein mosaic virus (BYVMV) (GU112057) and 89.7 % with okra yellow vein mosaic virus (OYVMV) (AJ002451) infecting okra in India and Indian subcontinent. These results suggested that the isolate is a new strain of BYVMV. To identify the resistance source to BYVMV, the okra genotypes were screened under both artificial and natural conditions. None of the genotypes showed immunity to the disease. However, the genotypes Nun 1145 and Nun 1144 showed moderate resistance and genotypes M10, Nun 1142, Nun 1140 showed moderately susceptible reactions under both glass house and field conditions. Further, dot-blot hybridization using nonradioactive (digoxigenin) DNA probe showed that the virus was also detected in the symptomless plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Okra (Abelmoschus esculentus L.) commonly known as bhendi or lady’s finger belongs to the Malvaceae family and is an important vegetable crop grown across different states of the country throughout the year. Among the different species of genus, Abelmoschus, the most popularly grown species is Abelmoschus esculentus in Asia and has great commercial demand due to its nutritional value. The major production constraint for okra is yellow vein mosaic disease, causing losses with regard to the quality and as well as the yield wherever the crop is grown. The yellow vein mosaic disease of okra (YVMD) is caused by Bhendi yellow vein mosaic virus (BYVMV) and was first reported in 1924 from the erstwhile Bombay Presidency (Kulkarni 1924). The virus belongs to the genus Begomovirus, family Geminiviridae (Fauquet and Stanley 2005). Recently, BYVMD complex was shown to be associated with the virus with a genomic component typical of monopartite begomoviruses, homologous DNA A and a single-stranded betasatellite (Jose and Usha 2003). This species is believed to have originated from India (Usha 2008) and its only known methods of transmission are through whitefly (Bemisia tabaci Gennadius) and grafting. The DNA A component has seven open reading frames encoding several multifunctional proteins involved in rolling circle replication, gene transcription, cell-to-cell and long-distance movement, suppression of host gene silencing, and encapsidation of the viral genome (Lazarowitz 1992). Betasatellites are approximately half the size of their helper begomoviruses required to induce typical disease symptoms in their original hosts (Briddon et al. 2001, 2003; Jose and Usha 2003). These satellites depend on their helper virus for replication, movement, encapsidation and vector transmission. The YVMD is characterized by a homogenous interwoven network of yellow vein enclosing islands of green tissue within its leaf. In extreme cases, infected leaves become completely yellowish or creamy. If plants are infected within 20 days after germination, their growth is retarded with few leaves and malformed fruits resulting in loss ranging from 94 to 100 % (Pun and Doraiswamy 1999). The extent of damage declines with delay in infection of the plants and was reported with a loss of 49–84 %, when infection occurred after 50–65 days of germination (Sastry and Singh 1974).
Further, the decline in the production of okra in India was attributed to several factors, such as loss of resistance to yellow vein mosaic in ruling varieties (Borah et al. 1992), emergence of new biotypes of whitefly vectors and development of moderate to strong resistance to commonly used insecticides by vectors (Rashida et al. 2005). At this stage, in order to implement sustainable pest management practices for the okra cropping system, there is a need to come out with tools, which will aid in quick identification of the virus/strains of begomovirus associated with yellow vein mosaic disease and to screen okra germplasm for YVMD resistance. With this backdrop, the current study was aimed at virus characterization and development of phenotypic and DNA-based diagnostics for screening germplasm to address this issue.
Materials and methods
Virus isolate and maintenance of Bemisia tabaci
A leaf sample from okra plants showing prominent yellow vein mosaic symptoms and two samples from non-symptomatic plants were collected from Chintamani, Karnataka state, India. The YVMD from the sample was whitefly transmitted to susceptible okra genotype (cv. 1685) and designated as virus isolate OYCHINT. Whitefly collection, maintenance and transmission were carried out as described by Venkataravanappa et al. (2012). After transmission, the inoculated plants were sprayed with an insecticide and maintained under insect-proof glasshouse for symptom expression. The plant tissues showing the symptoms were utilized for further analysis.
DNA isolation, PCR amplification, cloning and sequencing
Total nucleic acids were extracted from both non-symptomatic and symptomatic leaf tissues by the Cetyl trimethyl ammonium bromide method (Doyle and Doyle, 1990). The different components of virus genome were amplified by PCR as per the protocol and primers described by Venkataravanappa et al. (2012). The amplicons were cloned into the pTZ57R/T vector (Fermentas, Germany) according to the manufacturer’s instructions. The complete nucleotide sequence of the clones were determined by automated DNA sequencer ABI PRISM 3730 (Applied Biosystems) at Anshul Biotechnologies DNA Sequencing facility, Hyderabad, Andhra Pradesh, India. Three clones for each fragment were subjected to sequencing.
Comparison of DNA sequence
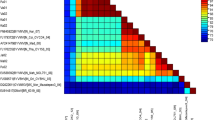
The similarity of genomic sequences was initially analyzed by using the BLAST program available at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/). The sequences (Table 1) showing highest scores with the present isolate were obtained from the database and multiple aligned using CLUSTAL-X program (Thompson et al. 1994). The sequence identity matrixes were generated using Bioedit Sequence Alignment Editor (version 5.0.9) (Hall, 1999) and phylogenetic tree was generated by MEGA 5.0 software (Tamura et al. 2011) using the neighbor joining method with 1,000 bootstrapped replications.
Plant material
The okra germplasm used for screening in the present study was the popularly grown tolerant variety/hybrid obtained from different sources. They are Arka Anamika, Pusa Sawani, cv1685, VRO-6, Punjab7, Hyb.218, HRB-107-4, AC1605(H5), NS 98, Nun 1144, Nun 1145, Nun 1142, Nun 1143, Nun 1140, M10, Kanchan and Indol 03-1. Apart from these some of the advanced breeding lines of okra viz; A.AXDJM-32, A.AX IIHR-1, DJM-32 X A. tetraphyllus, DJMA-3, IIHR-1XA.A, IIHR-233, IIHR-222, IC-141055, PKt3S3, PKt5S7, PKt6S6, PKt12S6 were collected from Division of vegetable breeding, Indian Institute of Horticulture Research, Bangalore, Karnataka.
Glasshouse screening of okra genotypes by whitefly inoculation
Inoculation of begomovirus by B. tabaci was conducted using cylindrical cages with mesh tops which were inverted over individual leaves. The insects were given access to YVMD-infected okra plants maintained in glass house in separate whitefly-proof cages. After acquisition access period of 24 h, the whiteflies were collected individually using an aspirator and transferred to separately caged test plants. Ten viruliferous adult whiteflies per each 1-week-old test plant were released and 24 h inoculation access period was given. After that the whiteflies were removed and plants were sprayed with 0.05 % imidacloprid insecticide and maintained in insect-proof screen house for symptom development. In each genotype, five plants were inoculated with nonviruliferous whiteflies, which were given acquisition access to healthy plants, which served as a control.
Natural screening of okra genotypes under field condition
A total of 20 genotypes of okra was screened for YVMD along with the susceptible check okra cultivar, 1685. For, every four rows of test genotype, one row of susceptible check were planted. Disease incidence was recorded and calculated using the formula below:

a is the number of diseased plants and b is the number of healthy plants.
Genotype classification
The okra varieties/hybrid/line were classified based on disease response to YVMD under both artificial and natural conditions using criteria previously described by Borah et al. (1992).
Grouping of plant response to infection of begomovirus.
S. no. | Disease incidence (%) | Plant response |
|---|---|---|
1 | 0.0 | Immune |
2 | X < 10 | Highly resistant (HR) |
3 | 10 < X > 20 | Resistant (R) |
4 | 20 < X > 30 | Moderately resistant (MR) |
5 | 30 < X > 50 | Moderately susceptible (MS) |
6 | 50 < X > 70 | Susceptible (S) |
7 | X > 70 | Highly susceptible (HS) |
DNA probe labeling, dot-blot hybridization and colorimetric detection
Coat Protein gene on homologous DNA A component of BYVMV (Isolate OYCHINT, Acc. No. GU112065) was used to design the probe. PCR amplification of coat protein gene was carried out with specific primer CPF and CPR and the amplified fragment was purified using the QIAquick gel extraction kit (QIAGEN Inc. Valencia, CA). The amplicon was then subjected for labeling using Random Primed Labeling with DIG-High Prime kit II (Roche diagnosis, Germany). Total nucleic acids were extracted from both symptomatic and non-symptomatic plants of different okra genotypes as described above. Total DNA of 20 μl from each sample was heated for 5 min at 100 °C on water bath and incubated at 4 °C before loading onto the membrane. After cooling, the DNA was loaded onto nitrocellulose membrane (Hybond-N, Amersham) using membrane loading commercial device (Dot Blot 96 System, Biometra, Germany). Then the membrane was air dried and DNA was cross-linked to the membrane by exposing to ultraviolet light (in a crosslinker device Amersham Pharmacia Biotech, USA). Different dilutions (10−1, 10−2, 10−3 and 10−4) of nucleic acid were used to check sensitivity of hybridization technique to detect the begomovirus in okra samples.
The pre-hybridization, hybridization and detection procedures were carried out according to the protocol given in DIG-High Prime DNA labeling and detection starter kit II (Roche diagnostics). Colorimetry-based detection was done with the use of nitroblue tetrazolium (NBT) and X-phosphate. Development of purple color at the location of sample on nitrocellulose membrane was indicative of positive reaction.
Results and discussion
PCR amplification, genome organization and sequence analysis
The complete genome component of virus isolate OYCHINT was amplified from the okra samples infected with YVMD collected from the field as well as glasshouse using three sets of primers. These primers were designed to amplify full genome in three fragments with approximately more than 200 bp overlapping to rule out mixed infections. Amplification with nucleic acid extracts from symptomless plants yielded no product. Positive amplification to betasatellite and failed to confirm the association of DNA B provided the evidence to conclude that, the current isolate under study is monopartite begomovirus.
The genome sequence of homologous DNA A component of the virus isolate was determined in both orientations and it was found to be 2,741 nucleotides in length and the sequence is available in the database under the accession number GU112065. The sequence had features, typical of Old World monopartite begomoviruses, with two open reading frames (ORFs) [AV1 (CP), AV2] in virion-sense strand and five ORFs [AC1 (Rep), AC2, AC3, AC4, AC5] in complementary-sense strand separated by an intergenic region (IR). In the IR region, the sequence identity of virus isolate was more than 90 % with IRs of BYVMV, for which a full-length sequence is available in the databases. The length of intergenic region (IR) is 298 nucleotides and encompasses an absolutely conserved hairpin structure containing nonanucleotide sequence (TAATATTAC) that marks the origin of virion-strand DNA replication and with repeated sequences known as “iterons” (GGAGTC) adjacent to the TATA box, which is the recognition sequence for binding of the rep to the promoter (Arguello-Astorga and Ruiz-Medrano 2001; Hanley-Bowdoin et al. 1999).
The comparison of genome sequence with the selected begomovirus sequences revealed that it shared highest sequence identity of 96.1 % with BYVMV (GU112057) and 89.7 % with OYVMV-(AJ002451) infecting okra in India and Indian subcontinent. This result was well supported by phylogenetic analysis with OYCHINT isolate closely clustering with BYVMV group (Fig. 1). Based on the current taxonomic criteria for begomovirus, the threshold cutoff of nucleotide identity for species demarcation is 89 % (Fauquet et al. 2008) and the virus isolates displaying more than this should be considered as strains rather than different virus species (Padidam et al. 1995). The present results indicate that OYCHINT is a new strain of Bhendi yellow vein mosaic virus from India infecting okra.
Phylogenetic trees constructed from aligned complete genome sequence (homologous DNA A component) of OYCHINT virus isolate with other begomoviruses by MEGA 5 using Neighbor-joining algorithm.Horizontal distances are proportional to sequence distances,vertical distances are arbitrary. The trees are unrooted. A bootstrap analysis with 1,000 replicates was performed and the bootstrap percent values more than 50 are numbered along branches
Evaluation of okra genotypes
Screening of germplasms under artificial condition
Totally, twenty-nine genotypes were screened under artificial condition by whitefly inoculation. The visual symptoms developed on different inoculated okra genotypes varied from yellow vein mosaic, vein thickening, petiole bending, venial chlorosis, intense yellowing, later turned brown, premature death and stunted growth of plant (Table 2). The disease incidence among different inoculated okra genotypes ranged from 5 to 100 %. Based on their susceptible reaction, the genotypes were classified as being resistant to highly susceptible by using criteria as previously described by Borah et al. (1992). None of the genotypes were immune to the virus. However, genotypes Nun 1145 and Nun 1144 showed moderate resistance reaction and symptoms appeared after 25–30 days of inoculation. Whereas, the genotypes Nun 1142, Nun 1140, and M10 showed moderately susceptible reaction and symptoms were produced after 15–25 days after the inoculation (Fig. 2a; Table 2). The genotypes Arka Anamika and Pusa sawani earlier showed to be resistant to BYVMV (Borah et al. 1992) were highly susceptible with much faster development of disease symptoms than other tested genotypes. The variation in symptoms in genotypes may be due to unique interaction between the particular virus strain and plant genotype or vector and genotype or altered feeding conditions of the vector (Polston and Anderson 1997; Delatte et al. 2006; Azizi et al. 2008). The begomoviruses transmitted by whitefly are directly deposited into phloem during salivation. Therefore, altered feeding behavior could result in a significant diminishing in the incidence of several begomoviruses that are usually interpreted as being resistant to insect vector (Parejarearn et al. 1984; Dintinger et al. 2005; Azizi et al. 2008).
Screening of germplasm under natural condition
Twenty okra genotypes were evaluated under field conditions with some advanced okra breeding lines. Three types of visual symptoms were observed on different okra genotypes. First type, the leaves of the young plants infected very early in the season became complete yellow and the leaves later turned brown and dried up. In the second type, plant infection started after flowering, upper leaves and flowering parts showed vein clearing symptoms. Infected plants produced some fruits but they became yellow and hard at picking stage. Third type, plants continued to grow in a healthy state and fruiting was normal till late in the season but, at the end, few small young shoots appeared at the basal portion of the stem, which showed only vein clearing. However, in such plants yield was as good as symptomless plants. This variation in symptoms under natural condition may be due to many factors like virus strain, time of infection, plant genotype, variation in biotypes of vector and their transmission and environmental factors (Polston and Anderson, 1997). The response of the varieties to virus infection under natural condition varied from incidence of 5 to 100 %. Further, their reaction of genotypes was classified similar to above. The genotypes Nun 1145 and Nun 1144 showed moderate resistance and genotypes Nun 1140, Nun 1142, Nun 1143 and M10 showed moderately susceptible reaction under natural condition (Fig. 2b; Table 3).
Based on the number of plants showing symptoms, both under artificial and natural conditions, cv. Nun 1144 and Nun 1145 were found to be moderately resistant, M10, Nun 1140, Nun 1142 and Nun 1143 were found to be moderately susceptible to the virus, whereas other genotypes were found to be susceptible to highly susceptible. Although completely resistant genotypes were not observed in this study, few genotypes which demonstrated tolerant-like responses to the virus infection can be utilized in breeding programmes. Certain genotypes such as Nun 1144 Nun 1145, M10, Nun 1140 and Nun 1142 have longer incubation period and fewer (<50 %) infected plants when inoculated with the virus. Similar results were observed when screening of different okra genotypes resistant to Bhendi yellow vein mosaic virus in the earlier studies (Dhankhar et al. 1996; Srivastava et al. 1995; Sannigrahi and Choudhury 1998; Batra and Singh 2000).
Dot-blot hybridization for detection of yellow vein mosaic virus
The non-radioactive digoxigenin-labeled DNA probe was used in dot-blot hybridization to detect the virus in the total DNA isolated from the symptomatic plant as well as non-symptomatic plant of different okra genotypes. The probe could be detected up to a concentration of 10−2 dilution in the plant showing the yellow vein mosaic disease (Fig. 3). The symptomless okra plants in all genotypes screened were also showed positive reaction. However, in certain genotypes the intensity of the reaction was less when compared to the plants expressing the symptoms. Based on the intensity of the reaction, the detection level could be differentiated into weak to strong reaction which is in turn indicative of virus titer in the host plants (Table 4; Fig. 4). Virus titer in plant tissue is an indicator of resistance or susceptibility of plants to the virus. Low levels of virus titer and decreasing virus accumulation rate in plant tissue indicate the presence of a resistance mechanism in the plant (Pico et al. 2001; Lapidot et al. 1997; Romero-Durban et al. 1993; Sharma et al. 2004). In the present study despite the high levels of similarities in symptom development in all the genotypes, there were considerable differences in BYVMV concentration in two genotypes (Nun 1144 and Nun 1145) both under artificial and natural conditions. Therefore, we tried to estimate the virus titer in both symptomatic and symptomless okra plants using digoxigenin-labeled DNA probe, the probe could detect begomovirus in both symptomatic and non-symptomatic plants (Table 4). Although radioactive methods have been widely used for several purposes including plant viral detection (Rodriguez et al. 2003), the introduction of non-radioactive probes has been necessary due to the environmental and technological disadvantages of the radioactive probes. Several authors have reported the use of the non-radioactive probe using markers such as digoxigenin, biotin and photobiotin, which are able to detect viral concentrations as low as compared to the radioactive probes (Singh et al. 1994; Li et al. 1995; Romero-Durban et al. 1993; Nakahara et al. 1998). Based on the outcome of the present study, it can be concluded that potential application of nonradioactive DNA probe for determining actual response of plant genotypes can be useful for routine large-scale diagnosis of geminiviruses affecting economically important crops in India.
References
Arguello-Astorga GR, Ruiz-Medrano R (2001) An iteron-related domain is associated to motif 1 in the replication proteins of geminiviruses: identification of potential interacting amino acid-base pairs by a comparative approach. Arch Virol 146:1465–1485
Azizi A, Javad M, Shams-bakhsh M (2008) Phenotypic and molecular screening of tomato germplasm for resistance to Tomato yellow leaf curl virus. Iran J Biotechnol 6:1999–2206
Batra VK, Singh J (2000) Screening of okra varieties to yellow vein mosaic virus under field conditions. Veg Sci 27:192–193
Borah GC, Saikia AK, Shadeque A (1992) Screening of okra genotypes for resistance to yellow vein mosaic virus disease. Indian J Virol 8:55–57
Briddon RW, Mansoor S, Bedford ID, Pinner MS, Saunders K, Stanley J, Zafar Y, Malik K, Markham PG (2001) Identification of DNA components required for induction of cotton leaf curl disease. Virology 285:234–243
Briddon RW, Bull SE, Amin I, Idris AM, Mansoor S, Bedford ID, Dhawan P, Rishi N, Siwatch SS, Abdel-Salam AM, Brown JK, Zafar Y, Markham PG (2003) Diversity of DNA beta: a satellite molecule associated with some monopartite begomoviruses. Virology 312:106–121
Delatte H, Holota H, Reynaud B, Dintinger J (2006) Characterisation of a quantitative resistance to vector transmission of Tomato yellow leaf curl virus in Lycopersicon pimpinellifolium. Eur J Plant Pathol 114:245–253
Dhankhar SK, Dhankhar BS, Saharan BS (1996) Screening of Okra genotypes for resistance to yellow vein mosaic disease. Ann Biol 12:90–92
Dintinger J, Boissot N, Chiroleu F, Hamon S, Reynaud B (2005) Evaluation of maize inbreds for maize strip virus and Maize mosaic virus resistance: disease progress in relation to time and accumulative number of planthoppers. Phytopathol 95:600–607
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fauquet CM, Stanley J (2005) Revising the way we conceive and name viruses below the species level: a review of geminivirus taxonomy calls for new standardized isolate descriptors. Arch Virol 150:2151–2179
Fauquet CM, Briddon RW, Brown JK, Moriones E, Stanley J, Zerbini M, Zhou X (2008) Geminivirus strain demarcation and nomenclature. Arch Virol 153:783–821
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids 41:95–98
Hanley-Bowdoin L, Settlage SB, Orozco BM, Nagar S, Robertson D (1999) Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Cri Rev Pl Sci 18:71–106
Jose J, Usha R (2003) Bhendi yellow vein mosaic disease in India is caused by association of a DNAβ satellite with a begomovirus. Virology 305:310–317
Kulkarni CS (1924) Mosaic and other related diseases of crops in the Bombay Presidency. Poona Agriculture College Magazine, Pune, p 16
Lapidot M, Friedmann M, Lachman O, Yehezkel A, Nahon S, Cohen S, Pilowsky M (1997) Comparison of resistance level to tomato yellow leaf curl virus among commercial cultivars and breeding lines. Plant Disease 81:1425–1428
Lazarowitz SG (1992) Geminiviruses: genome and structure and gene function. Rev Plant Sci. 11:327–349
Li SF, Onodera S, Sano T, Yoshida K, Wang GP, Shikata E (1995) Gene diagnosis of viroid: comparisons of return-PAGE and hybridization using DIG-labelled DNA and RNA probes for practical diagnosis of Hop Stunt, Citrus Exocortis and Apple Scar Skin viroids in their natural host plants. Ann Phytopatol Soc Jpn 61:381–390
Nakahara KHT, Hataya T, Sugimoto T, Kimura I, Shigata E (1998) A mixture of synthetic probes labelled with biotin for the sensitive detection of potato spindle tuber viroid. J Virol Methods 71:219–227
Padidam M, Beachy RN, Fauquet CM (1995) Tomato leaf curl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J Gen Virol 76:25–35
Parejarearn A, Lapis DB, Hibino H (1984) Reaction of rice varieties to rice ragged stunt virus (RSV) infection by three brown planthopper (BPH) biotypes. Int Rice Res Newsl 9:7–8
Pico B, Ferriol M, Diaz MJ, Vinals FN (2001) Agroinoculation method to screen wild Lycopersicon for resistance to Tomato yellow leaf curl virus. J Plant Pathol 83:215–220
Polston JE, Anderson PK (1997) The emergence of whitefly-transmitted geminiviruses in tomato in Western Hemisphere. Plant Disease 81:1358–1369
Pun KB, Doraiswamy S (1999) Screening of plant species for the presence of antiviral principles against Okra yellow vein mosaic virus. Indian Phytopath 52:221–223
Rashida P, Sultan MK, Khan MA, Noor-Ul-Islam (2005) Screening of cotton germplasm against cotton leaf curl Begomovirus (CLCuV). J Agri Soc Sci 3:35–238
Rodriguez R, Ramos PL, Vivian D, Velazquez K, Rudy P, Fuentes A, Pujol M (2003) Establishment of a non-radioactive nucleic acid hybridization technique for begomovirus detection. Biotecnologia Aplicada 20:164–169
Romero-Durban M, Antignus Y, Gidoni D, Pilowsky M, Cohen S (1993) Accumulation of tomato yellow leaf curl virus DNA in tolerant and susceptible tomato lines. Plant Dis 77:253–257
Sannigrahi AK, Choudhury K (1998) Evaluation of okra cultivars for yield and resistance to yellow vein mosaic virus in Assam. Environ Ecol 16:238–239
Sastry KSM, Singh SJ (1974) Effect of yellow vein mosaic virus infection on growth and yield of okra crop. Indian Phytopath 27:294–297
Sharma P, Rishi N, Malathi VG (2004) Nucleic acid probe based technique for detection of cotton leaf curl virus in India. Indian J Biotech 3:133–135
Singh RP, Boucher A, Laksman DK, Tavantzis SM (1994) Multimeric non-radioactive cRNA probes improve detection of potato spindle tuber viroid (PSTVd). J Virol Methods 49:221–234
Srivastava PK, Srivastava KJ, Sharma HK, Gupta RP (1995) Evaluation of different varieties of okra against yellow vein mosaic virus (YVMV). Nat Hort Res Dev Foundation Newsl 15:8–10
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 10:1093
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Usha R (2008) Bhendi yellow vein mosaic virus. In: Rao GP, Kumar PL, Holguın-Pena RJ (eds) Characterization, diagnosis & management of plant viruses, vol 3. Studium Press, Houston, pp 387–392
Venkataravanappa V, Reddy CNL, Jalali S, Krishna Reddy M (2012) Molecular characterization of distinct bipartite begomovirus infecting bhendi (Abelmoschus esculentus L.) in India. Virus Genes 44(3):522–535
Acknowledgments
The research was supported by ICAR NETWORK project on development of diagnostics to emerging plant viruses, Indian Council of Agricultural Research, Government of India, New Delhi. We are thankful to Dr.M. Pitchaimuthu, Principal Scientist, Division of vegetable Crops, IIHR, Bangalore for providing us the okra genotypes. We are thankful to Dr. H.C. Prasanna, Division of crop improvement, Indian Institute Vegetable Research, India for his suggestions in manuscript preparation.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Venkataravanappa, V., Lakshminarayana Reddy, C.N. & Krishna Reddy, M. Begomovirus characterization, and development of phenotypic and DNA-based diagnostics for screening of okra genotype resistance against Bhendi yellow vein mosaic virus. 3 Biotech 3, 461–470 (2013). https://doi.org/10.1007/s13205-012-0107-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13205-012-0107-z