Abstract
1-Methyl-1,2,3,4-tetrahydroisoquinoline (1MeTIQ) is present in the human and rodent brain as a mixture of stereospecific (R)- and (S)-1MeTIQ enantiomers. The racemate, (R,S)-1MeTIQ, exhibits neuroprotective activity as shown in the earlier study by the authors, and In addition, it was suggested to play a crucial physiological role in the mammalian brain as an endogenous regulator of dopaminergic activity. In this article, we investigated the influence of stereospecific enantiomers of 1MeTIQ, (R)- and (S)-1MeTIQ (50 mg/kg i.p.) on rotenone-induced (3 mg/kg s.c.) behavioral and neurochemical changes in the rat. In behavioral study, in order to record dynamic motor function of rats, we measured locomotor activity using automated locomotor activity boxes. In biochemical studies, we analyzed in rat striatum the concentration of dopamine (DA) and its metabolites: intraneuronal DOPAC, extraneuronal 3-MT, and final HVA using HPLC with electrochemical detection. Otherwise, DA release was estimated by in vivo microdialysis study. The behavioral study has demonstrated that both acute and repeated (3 times) rotenone administration unimportantly depressed a basic locomotor activity in rat. (R)- and (S)-1MeTIQ stereoisomers (50 mg/kg i.p.) produced a modest behavioral activation both in naïve and rotenone-treated rats. The data from ex vivo neurochemical experiments have shown stereospecificity of 1MeTIQ enantiomers in respect of their effects on DA catabolism. (R)-1MeTIQ significantly increased both the level of the final DA metabolite, HVA (by about 70%), and the rate of DA metabolism (by 50%). In contrast to that, (S)-1MeTIQ significantly depressed DOPAC, HVA levels (by 60 and 40%, respectively), and attenuated the rate of DA metabolism (by about 60%). On the other hand, both the enantiomers increased the concentrations of DA and its extraneuronal metabolite, 3-MT in rat striatum. In vivo microdialysis study has shown that repeated but not acute administration of rotenone produced a deep and significant functional impairment of striatal DA release. Both (R)- and (S)- stereospecific enantiomers of 1MeTIQ antagonized rotenone-induced suppression of DA release; however, the effect of (R)-1MeTIQ was more strongly expressed in microdialysis study. In conclusion, we suggest that both chiral isomers of 1MeTIQ offer neuroprotection against rotenone-induced disturbances in the function of dopaminergic neurons and (R,S)-1MeTIQ will be useful as a drug with marked neuroprotective activity in the brain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurological disorder, and the most of its cases are idiopathic in nature even though in the last few decades, many familial cases have been described as well, and in some of them the underlying genetic defect has been identified. Parkinson`s disease is caused by mitochondrial complex I deficiency and neurodegeneration of dopaminergic neurons in the substantia nigra (Schapira et al. 1990). At present, it is believed that both genetic and environmental factors are responsible for Parkinson`s disease. Its classical cardinal signs include rigidity and bradykinesia, resting tremor, and postural instability.
The environmental neurotoxins may largely contribute to the development of this illness. One of the most famous toxins of dopaminergic neurons is 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) which after injection to humans and animals produces rapid, irreversible parkinsonism (Langston et al. 1983; Bankiewicz et al. 1986). MPTP, acting through its metabolite, 1-methyl-4-phenylpyridinium ion (MPP+), induces degeneration of dopaminergic neurons, predominantly in the substantia nigra. It was believed that the main mechanism by which MPP+ caused neuronal damage involved mitochondrial dysfunction produced by the inhibition of mitochondrial complex I activity that led to mitochondrial depolarization and generation of reactive oxygen species (Nakamura et al. 2000). However, Lotharius and O’Malley (2000) indicated that the generation of reactive oxygen species during the oxidation of dopamine released by MPP+ was the main consequence of MPP+-induced neurotoxicity. MPTP has frequently been used to provide an animal model of Parkinson`s disease (Gerlach and Riederer 1996).
More recently, it has been found that another, much more frequently encountered environmental toxin, rotenone may be used to produce a more realistic animal model of Parkinson`s disease (Betarbet et al. 2000). Rotenone, a natural compound, is a classical lipophilic inhibitor of mitochondrial complex I (Gutman et al. 1970; Horgan et al. 1968), and is selectively toxic to dopaminergic neurons (Marey-Semper et al. 1993; Testa et al. 2005). Injected directly into brain structures, rotenone acts similarly to MPTP (Heikkila et al. 1985). Rotenone is the only neurotoxin known today that induces the formation of Lewy bodies, which are the most characteristic histopathological feature of Parkinson’s disease (Betarbet et al. 2000). As in the case of MPTP, a defect of mitochondrial function due to complex I inhibition was postulated to be the cause of rotenone-induced neurodegeneration (Betarbet et al. 2000; Jenner 2001; Greenamyre et al. 2001). Rotenone used in a high concentration also causes dopamine release, as evidenced by microdialysis and neurochemical data (Santiago et al. 1995; Thiffault et al. 2000), and this may also contribute to the degeneration of dopaminergic neurons. Chronic administration of a low dose of rotenone in rats reproduces most of the motor, neurochemical, and pathological features of PD shown to be attenuated by L-DOPA (Alam and Schmidt 2002).
In addition to the exogenous neurotoxins which have been widely used as convenient and acceptable models for the induction of experimental Parkinson`s disease, endogenous amines present in the human and animal brain, such as salsolinol or 1-benzyl-1,2,3,4-tetrahydroisoquinoline (1BnTIQ) and to a lesser extent 1,2,3,4-tetrahydroisoquinoline (TIQ) (Lorenc-Koci et al. 2000, 2004) may participate in the pathogenesis of Parkinson`s disease (Abe et al. 2005; Antkiewicz-Michaluk et al. 2000a, b; Antkiewicz-Michaluk and Vetulani 2001; Kotake et al. 1995, 1996; Nagatsu 1997; Niwa et al. 1987). However, among them TIQ, and its close methyl derivative, 1-methyl-1,2,3,4-tetrahydroisoquinoline (1MeTIQ) was found to be a neuroprotective (Antkiewicz-Michaluk et al. 2006; Lorenc-Koci et al. 2000, 2005; Kohno et al. 1986; Tasaki et al. 1991; Yamakawa and Ohta 1999; Yamakawa et al. 1999) and antiaddictive compound (Antkiewicz-Michaluk et al. 2005, 2007; Filip et al. 2007; Wąsik et al. 2007; Wróbel 2005) with special effects on dopamine metabolism (Antkiewicz-Michaluk et al. 2001, 2006).
More recently, our experiments have shown a neuroprotective effect of 1MeTIQ evidenced by the reduction of rotenone-induced mortality and changes in dopamine metabolism, and finally, by mitigation of dopamine nigrostriatal neuron degeneration after rotenone injection into the medial forebrain bundle (Antkiewicz-Michaluk et al. 2003, 2004). It is important to mention that 1MeTIQ is present in the human and rodent brain as a mixture of stereospecific (R)- and (S)-1MeTIQ enantiomers. The racemate, (R,S)-1MeTIQ has been found to restraint the formation of 3,4-dihydroxyphenylacetic acid (DOPAC), during dopamine metabolism and to shift the catabolism toward the O-methylation route, while the neurotoxic substances like: 1-benzyl-1,2,3,4-tetrahydroisoquinoline, rotenone or MPTP produce the opposite effect (Antkiewicz-Michaluk et al. 2001; Antkiewicz-Michaluk and Vetulani 2001; Antkiewicz-Michaluk et al. 2003; Lotharius and O’Malley 2000). In addition, (R,S)-1MeTIQ, was shown to prevent MPTP-, 1BnTIQ- and rotenone-induced behavioral and neurochemical abnormalities (Antkiewicz-Michaluk et al. 2003, 2004; Kotake et al. 1995; Tasaki et al. 1991). Furthermore, it was demonstrated that (R,S)-1MeTIQ was markedly depleted in the parkinsonian human brain (Ohta et al. 1987). Thus, (R,S)-1MeTIQ is considered to play an important role in the prevention of neurotoxin-induced parkinsonism. However, the question arises whether the chirality of 1MeTIQ affects these phenomena, and which of these two enantiomers expresses a neuroprotective activity. In addition, recent regulatory guidelines have recommended the development of single enantiomer of compounds that have or may have clinical applications. This recommendation is based on the idea that a single enantiomer may offer clinical advantages over the racemate in terms of potency, efficacy and tolerability. In order to address these problems, we decided to study the effects of the stereo-structure of 1MeTIQ using the (R)- and (S)-1MeTIQ in rotenone-treated rats. In the last decade, it has been shown that systemic administration of rotenone induces different patterns of dopamine system abnormalities depending on the route of administration, dose regimen used and the animal species.
In this study, we aim to show the first signs of the functional impairment of dopamine system produced by a low dose (3 mg/kg) of repeated intraperitoneal rotenone administration. For the estimation of the dopaminergic system function, we used both ex vivo an in vivo biochemical studies, and compared the effects of an acute and multiple (three times) injection of rotenone on dopamine metabolism and its in vivo release in rat striatum. It is important to mention that such experimental design did not produce yet the depletion of tissue dopamine concentration in the striatum although, the function of dopamine neurons was already disturbed. In addition, to record dynamic behavior of rats we measured also locomotor activity using automated locomotor activity boxes. Finally, we investigated the possible neuroprotective role of optically active 1MeTIQ enantiomers: (R)- and (S)-1MeTIQ in the rotenone-induced disturbances in the function of striatal dopamine neurons.
Materials and Methods
Animals and Treatment
Behavioral tests were carried out on male Wistar (Charles River) rats, of initial body weight 260–300 g (about 9 weeks old) kept under standard laboratory conditions, eight to a large animal cage. The rats used for the microdialysis study after surgery were housed for a couple of days individually. All animals had free access to standard laboratory food and tap water, and were maintained at room temperature (22°C) with natural day–night cycle. The experiments were carried out between 09:00 and 16:00 h. Control rats were treated with an appropriate solvent.
Acute and multiple rotenone injections were administered subcutaneously (s.c.) in a low, 3 mg/kg dose, as a suspension prepared in 1% Tween 80 solution by sonication. The (R)- and (S)-1MeTIQ enantiomers were dissolved in 0.9% NaCl solution and administered in a dose of 50 mg/kg intraperitoneally (i.p.). In the combined groups, (R)- and (S)-1MeTIQ enantiomers were administered only once, 5 min before a single dose of rotenone or in the case of repeated rotenone treatments just before the third injection of rotenone. The dose of both (R)- and (S)-1MeTIQ enantiomers was chosen based upon their activity in the in vivo functional and biochemical studies (Antkiewicz-Michaluk et al. 2009; Wasik et al. 2009a, b).
All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were granted an approval from the Bioethics Commission as compliant with Polish Law. All the experimental procedures were approved by the Local Bioethics Commission of the Institute of Pharmacology, Polish Academy of Sciences in Kraków.
Drugs
Rotenone (Sigma, St. Louis, MO USA) was obtained commercially. (R)- and (S)-enantiomers of 1-methyl-1,2,3,4-tetrahydroisoquinoline (1MeTIQ), were synthesized by Dr. Jan Boksa, Department of Drug Chemistry, Institute of Pharmacology Polish Academy of Sciences, Krakow, Poland. Purity of the compounds was verified by measurement of the melting point, and homogeneity was assessed on a chromatographic column. The stereochemical structures of the (R)- and (S)-1MeTIQ are shown in Fig. 1.
Behavioral Tests
Locomotor Activity
The locomotor activity and rearing were examined using actometers Opto-Varimex activity monitors (Columbus Inst., USA) linked on line to an IBM-PC compatible computer. Each cage (43 × 44 × 25 cm) was surrounded with a 15 × 15 array of photocell beams located 3 cm from the floor surface as reported previously (Filip et al. 2007). Interruptions of these photocell beams were counted as a measure of horizontal-locomotor activity defined as the distance travelled (in cm). Each data expressed of unit time (15 min).
The rats received rotenone in a dose of 3 mg/kg s.c. acutely or for three consecutives days, the control group was treated with the solvent. (R)- and (S)- 1MeTIQ enantiomers (50 mg/kg i.p.) were administered acutely, and in the combined groups 5 min before acute rotenone administration or just before the third rotenone injection. Afterward, the animals were transferred to the experimental cages, and after a 30-min adaptation the basic locomotor activity (travelled distance in cm) was recorded during 90 min. The results were analyzed using Auto-Track Software Program (Columbus Instruments, USA). From five to six animals per group were used in behavioral study. After completion of the behavioral test, the rats were decapitated (3 h after rotenone injection), and the striatum was removed for further biochemical evaluation.
Biochemical Assays
Ex vivo Biochemical Studies—Dopamine Metabolism in the Rat Striatum
At the end of the behavioral experiments, the rats were killed by decapitation, and the striatum was dissected immediately and the obtained tissue was frozen on solid CO2 (−70°C) and stored until used for biochemical assay. Dopamine and its metabolites, the intraneuronal metabolite, DOPAC; the extraneuronal-3-methoxytyramine (3-MT); and the final metabolite, homovanillic acid (HVA) were assayed by means of high-performance liquid chromatography (HPLC) with electrochemical detection. The chromatograph HP 1050 (Hewlett-Packard, USA) was equipped with Hypersil columns BDS-C18 (4 × 100 mm, 3 μm). The tissue samples were weighed and homogenized in an ice-cold 0.1 M perchloroacetic acid containing 0.05 mM ascorbic acid. After centrifugation (10,000 × g, 5 min), the supernatants were filtered through RC58 0.2-μm diameter of pore cellulose membranes (Bioanalytical Systems, West Lafayette, IN, USA). The mobile phase consisted of 0.05 M citrate–phosphate buffer, pH 3.5, 0.1 mM EDTA, 1 mM sodium octyl sulfonate and 3.5% methanol. The flow rate was maintained at 1 ml/min. Dopamine and its metabolites were quantified by peak area comparisons with standards run on the day of analysis.
In vivo Dopamine Release in the Rat Striatum—a Microdialysis Study
The rats were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg) and secured in stereotaxic frame (Stoelting, USA). Vertical microdialysis guides (Intracerebral Guide Cannula with stylet; BAS Bioanalitical, USA) were implanted in the striatum (STR) with the following stereotaxic coordinates: A/P +1.0, L/M +2.5, and V/D −3.5 mm from the bregma point and dura, respectively (G. Paxinos and CH Watson).
On the seventh day after surgery, the microdialysis probes were placed inside the guides, and next the striatum was perfused with an artificial cerebrospinal fluid (aCSF) consisting of 140 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, 0.3 mM NaH2PO4, 1.7 mM Na2HPO4, pH 7.4 at a flow rate of 1.5 μl/min with a microinfusion pump (Stoelting, Illinois USA). The samples were collected from freely moving rats at 20-min intervals after a 3-h wash-out period. The rats received rotenone in a dose of 3 mg/kg s.c. acutely or repeatedly (for 3 consecutives days), and the control group was treated with the vehicle. The basal samples were collected from “−60” to “0” (control samples), and in the case of repeated treatment of rotenone the basal samples “−60’ to “0” were collected 24 h after the second injection of rotenone just before the third its injection in the point “0”. (R)- and (S)- 1MeTIQ enantiomers (50 mg/kg i.p.) were administered acutely, and in the combined groups together with acute rotenone administration or just before the third rotenone injection (repeated treatment). The dialysate was collected every 20 min up to 3 h. All probes were immediately frozen on solid CO2 (−70°C) till used for biochemical assay.
Dopamine (DA) was assayed in dialysates (20 μl) using HPLC with ESA Coulochem III elecrochemical detection (USA). The chromatograph Dionex (Germany) was equipped with Hypersil Gold columns C18 (3 × 150 mm, 3 μm). The mobile phase consisted of 0.05 M citrate–phosphate buffer, pH 3.5, 0.1 mM EDTA, 1 mM sodium octyl sulfonate and 3.5% methanol. The flow rate was maintained at 1 ml/min. The chromatographic data were processed by ChemStation computer program and dopamine was quantified by peak area comparisons with standard run on the day of analysis.
At the end of the experiments, brains were examined histologically in frozen slices for verification of probe placement (Fig. 2). Six animals per group were used for microdialysis study.
Calculations and Statistics
The results from behavioral studies were calculated by a two-way analysis of variance (ANOVA) for repeated measures, followed when appropriate by Duncan’s post-hoc test. The results were considered statistically significant when P < 0.05.
Ex vivo biochemical experiments were analyzed by means of a two way-analysis of variance (ANOVA) followed by Duncan’s post-hoc test when appropriate. The results were considered statistically significant when P < 0.05. The total catabolism rate for dopamine was assessed from the ratio of the final dopamine metabolite concentration, homovanillic acid to dopamine concentration and expressed as the catabolic rate index ([HVA]/[DA]) × 100. The index of the oxidative dopamine catabolism rate (MAO dependent) was expressed as ([DOPAC]/[DA]) × 100, and the index of the O-methylation dopamine catabolism rate (COMT dependent) as ([3-MT]/[DA]) × 100. The indices were calculated using concentrations from individual tissue samples as described before (Antkiewicz-Michaluk et al. 2001).
The results from microdialysis in vivo studies were analyzed by means of a repeated one-way analysis of variance (ANOVA) followed by Duncan’s post-hoc test when appropriate. The results were compared to 100% of basal line which is defined as the mean concentration of −60 to 0 time. The results were considered statistically significant when P < 0.05.
Results
Behavioral Tests
Locomotor activity: the effect of (R)- and (S)-1MeTIQ enantiomers.
Acute Rotenone
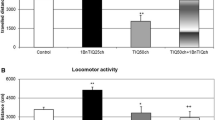
The two-way ANOVA for repeated measures has shown no significant effect of acute rotenone administration on the locomotor activity (Fig. 3a, b) in rats. On the other hand, the repeated two-way ANOVA revealed a significant effect of both, (R)- and (S)-1MeTIQ enantiomers on the rat locomotor activity as follow: Treatment F 1,19 = 15.52, P < 0.0002; F 1,19 = 20.12, P < 0.0002, respectively, and Time F 5,95 = 3.47, P < 0.006; F 5,95 = 3.54, P < 0.005, respectively (Fig. 3a, b). The effect of Time versus Treatment of (S)-1MeTIQ enantiomer was also significant (F 5,95 = 2.50, P < 0.035). Post-hoc analysis indicated a significant elevation of the rat locomotor activity after both (R)- and (S)-1MeTIQ enantiomer administration in naive and in rotenone-treated rats (Fig. 3a, b).
Locomotor activity (horizontal activity) after a single administration of rotenone (3 mg/kg s.c.), (R)-and (S)-1MeTIQ enantiomers, and combined treatment in rat. a The effect of (R)-1MeTIQ (50 mg/kg i.p.) enantiomer. b The effect of (S)-1MeTIQ (50 mg/kg i.p.). The number of animals in a group n = 4–6. The data are expressed as means ± S.E.M. * P < 0.05, ** P < 0.01 to Control group; + P < 0.05, ++ P < 0.01 to Rotenone-treated group (two-way analyses of variance ANOVA for repeated measures, followed when appropriate by Duncan’s post-hoc test)
Repeated Rotenone
The repeated (three consecutive days) administration of rotenone similarly to the acute injection did not reveal a significant effect (a repeated two-way ANOVA) on the locomotor activity (Fig. 4a, b). The two-way ANOVA for repeated measures has shown a significant effect of Treatment by (R)- and (S)-1MeTIQ enantiomers (F 2,18 = 7.55, P < 0.0041) and Time (F 7,126 = 71.90, P < 0.00001) on the rat locomotor activity. The effect of Time versus Treatment was also significant (F 14,126 = 3.22, P < 0.0002). As demonstrated by post-hoc analysis, (R)- and (S)-1MeTIQ enantiomers produced a significant elevation of locomotor activity both in naive and repeated rotenone-treated rats (Fig. 4a, b).
Locomotor activity (horizontal activity) after repeated rotenone administration (three consecutive days; 3 mg/kg s.c.). a The effect of a single administration of (R)-1MeTIQ (50 mg/kg i.p.) in repeatedly rotenone-treated rat. b The effect of a single administration of (S)-1MeTIQ (50 mg/kg i.p.) in repeatedly rotenone-treated rat. The number of animals in a group n = 4–6. The data are expressed as means ± S.E.M. * P < 0.05, ** P < 0.01 to Control group; + P<0.05, ++ P < 0.01 to Rotenone-treated group (two-way analyses of variance ANOVA for repeated measures, followed when appropriate by Duncan’s post-hoc test)
Biochemical Study
Ex vivo Studies
The analysis showed a general Treatment effects for acute and repeated Rotenone (3 mg/kg s.c.), 1MeTIQ (R)-and (S)-enantiomers (50 mg/kg i.p.), and Interaction between Rotenone × 1MeTIQ enantiomers on the concentration of DA, and its metabolites: DOPAC, 3-MT, HVA. In addition, the dopamine catabolism rate was presented as the following ratios: total ([HVA]/[DA]); oxidation ([DOPAC/[DA]) and O-methylation ([3-MT/[DA]) in the rat striatum, and demonstrated by two-way ANOVA analysis of variance (Tables 1, Table 2).
Acute Rotenone (Table 1)
A two-way ANOVA revealed a non-significant effects of Treatment 1 with Rotenone and Interaction of Rotenone × 1MeTIQ enantiomers on all investigated dopaminergic parameters. On the other hand, Treatment 2 with 1MeTIQ enantiomers has shown a significant effects on the concentration of DOPAC (F 2,20 = 241.58, P < 0.00001), 3-MT (F 2,20 = 22.16, P < 0.00005), HVA (F 2,20 = 98.97, P < 0.00001), and catabolic ratios: total (F 2,20 = 96.31, P < 0.00001), oxidation (F 2,20 = 242.35, P < 0.00001), O-methylation (F 2,20 = 32.75, P < 0.00001) in the rat striatum (Table 1). A subsequent Duncan`s post-hoc analysis revealed that concentration of the intraneuronal DA metabolite, DOPAC was considerably decreased in the (S)-1MeTIQ and Rotenone + (S)-1MeTIQ-treated groups (by about 70% of the control, P < 0.01). The content of the extraneuronal DA metabolite, 3-MT was significantly elevated in (R)- and (S)-1MeTIQ-treated groups as well as in jointed treatments of 1MeTIQ enantiomers with Rotenone (by about 40–85% of the control, P < 0.01). The level of the final DA metabolite, HVA was markedly elevated in (R)-1MeTIQ group (by about 50% of the control, P < 0.01) and in combined (R)-1MeTIQ + Rotenone group (by 70% of the control, P < 0.01). In contrast to that, in (S)-1MeTIQ-treated group as well as in joined (S)-1MeTIQ + Rotenone group the concentration of HVA was strongly decreased (by about 50% of the control, P < 0.01).
A two-way ANOVA demonstrated a significant effect (F 2,20 = 96.31, P < 0.00001), of Treatment 2 with 1MeTIQ enantiomers on the total DA metabolism rate expressed as the ratio of ([HVA]/[DA]) in the striatum. A post-hoc analysis revealed a significantly increased DA metabolism rate in (R)-1MeTIQ and (R)-1MeTIQ + Rotenone-treated groups (by about 40% of the control, P < 0.01). In contrast to that, in (S)-1MeTIQ-treated groups, an appreciably decreased DA metabolism rate (by about 40% of the control, P < 0.01) was observed. The rate of the oxidation pathway of DA catabolism expressed as ([DOPAC]/[DA]) was strongly decreased in (S)-1MeTIQ-treated group (by about 75% of the control value) whereas in (R)-1MeTIQ-treated groups the effect was weak (a decrease by about 15% of the control, P < 0.05). The rate of O-methylation pathway of DA catabolism was significantly elevated ( from 25% P < 0.05 to 70% P < 0.01 of the control) in all groups treated with 1MeTIQ enantiomers (Table 1).
Repeated Rotenone (Table 2)
A two-way ANOVA demonstrated a non-significant effects of Treatment 1 with repeated Rotenone and Interaction of repeated Rotenone × 1MeTIQ enantiomers on the concentration of DA and its metabolites in the rat striatum. Treatment 1 with repeated Rotenone revealed only a significant effect on the DA O-methylation rate ([3-MT]/[DA]) (F 1,22 = 16.85, P < 0.000467). On the other hand, Treatment 2 with 1MeTIQ enantiomers has shown a significant effects on all investigated biochemical parameters: concentration of DA (F 2,22 = 3.75, P < 0.039758); DOPAC (F 2,22 = 41.51, P < 0.00001); 3-MT (F 2,22 = 26.87, P < 0.00001); HVA (F 2,22 = 47.07, P < 0.00001); and the catabolic ratios: total ([HVA]/[DA]) (F 2,22 = 144.00, P < 0.00001), oxidation ([DOPAC]/[DA]) (F 2,22 = 199.95, P < 0.00001), O-methylation ([3-MT]/[DA]) (F 2,22 = 50.805, P < 0.00001) in the rat striatum (Table 2). A subsequent Duncan`s post-hoc analysis revealed that concentration of the intraneuronal DA was considerably increased in the (R)- and (S)-1MeTIQ-treated groups (by about 30% of the control, P < 0.05) and in Rotenone + (S)-1MeTIQ-treated group (by about 27% of the control, P < 0.05). The content of the intraneuronal DA metabolite, DOPAC was significantly elevated by (R)-1MeTIQ-treated group (by 30% of the control, P < 0.026), and opposite to that DOPAC was decreased in (S)-1MeTIQ and Rotenone + (S)-1MeTIQ-treated groups (by about 50% to 60% of the control, respectively, P < 0.001). The content of the extraneuronal DA metabolite, 3-MT was significantly depressed (by about 20%, P < 0.05) in repeated Rotenone-treated group, and opposite to that elevated in (R)- and (S)-1MeTIQ-treated groups as well as in jointed treatments of 1MeTIQ enantiomers with Rotenone (by about 25% to 130% of the control, P < 0.004). The level of the final DA metabolite, HVA was markedly elevated in (R)-1MeTIQ group (by about 130% of the control, P < 0.0001) and in combined (R)-1MeTIQ + Rotenone group (by 80% of the control, P < 0.001). In contrast to that, in (S)-1MeTIQ--treated group as well as in joined Rotenone + (S)-1MeTIQ-treated group the concentration of HVA was decreased (by about 45% of the control, P < 0.05) (Table 2).
A two-way ANOVA demonstrated a significant effect (F 2,22 = 144, P < 0.00001) of 1MeTIQ enantiomer treatments (Treatment 2) but not repeated Rotenone treatment (Treatment 1) on the total DA metabolism rate expressed as the ratio of ([HVA]/[DA]) in the striatum. A post-hoc analysis revealed a significantly increased DA metabolism rate in (R)-1MeTIQ and Rotenone + (R)-1MeTIQ-treated groups (by about 70% of the control, P < 0.0001). In contrast to that, in (S)-1MeTIQ-treated groups an appreciably decreased DA metabolism rate (by from about 45% to 60% of the control, P < 0.0005) was observed. The rate of the oxidation pathway of DA catabolism expressed as ([DOPAC]/[DA]) was strongly decreased in (S)-1MeTIQ-treated groups (by about 65% of the control value, P < 0.0005) whereas in (R)-1MeTIQ-treated groups it was not changed. A two-way ANOVA demonstrated a significant effect (F 1,22 = 16.848, P < 0.00046) of repeated Rotenone treatment (Treatment 1) as well as 1MeTIQ enantiomers treatments (Treatment 2) (F 2/22 = 50.805, P < 0.0001) on the rate of O-methylation pathway of DA catabolism in the rat striatum. A post-hoc analysis revealed that repeated Rotenone treatment produced a significant decrease the rate of DA O-methylation (by about 25% of the control, P < 0.05). In contrast to that both 1MeTIQ enantiomers increased the rate of DA O-methylation (by about 40% to 80% of the control, P < 0.01) and completely antagonized the effect of Rotenone (Table 2).
In Vivo Dopamine Release in the Rat Striatum—a Microdialysis Study
Acute Rotenone
Mean control basal extracellular concentration of dopamine in dialysates from the striatum was about 9.8 ± 1.3 (pg/20 μl). A repeated-measures analysis of variance revealed not significant effect of Treatment (with acute Rotenone, (R)-1MeTIQ and combined group Rotenone + (R)-1MeTIQ) but significant effect of Time (F 2,108 = 4.778, P < 0.00001) and interaction of Time versus Treatment (F 2,108 = 2.139, P < 0.0043). A post-hoc analysis demonstrated not significant effect of Rotenone and (R)-1MeTIQ enantiomer, and only a slight but significant increase of dopamine release in the striatum in the combined Rotenone + (R)-1MeTIQ-treated group (Fig. 5a). In case of (S)-1MeTIQ enantiomer, a repeated-measures analysis of variance has shown a significant effect of Treatment (with acute Rotenone, (S)-1MeTIQ and co-administered group Rotenone + (S)-1MeTIQ) (F 2,108 = 6.870, P < 0.0154) as well as Time (F 2,108 = 16.609, P < 0.00001) and interaction of Time versus Treatment (F 2,108 = 4.127, P < 0.00001). A post-hoc Duncan’s test demonstrated that (S)-1MeTIQ enantiomer-treated group as well as joined Rotenone + (S)-1MeTIQ group showed a significantly increased (about 230%, P < 0.01) dopamine release in the rat striatum. Opposite to that, acute Rotenone administration did not affect dopamine release in the striatum (Fig. 5b).
Microdialysis study: In vivo dopamine release in rat striatum after acute administration of rotenone (3 mg/kg s.c.). a The effect of (R)-1MeTIQ (50 mg/kg i.p.). b The effect of (S)-1MeTIQ (50 mg/kg i.p.). The basal value from the point “−60” to “0”. The arrow in the point “0” indicates the drug injections. The number of animals in a group n = 4. The data are expressed as means ± S.E.M. * P < 0.05, ** P < 0.01 versus the basal value (one-way analyses of variance ANOVA followed by Duncan’s post-hoc test when appropriate)
Repeated Rotenone
The comparison of the effects of the acute and repeated (3 days) Rotenone administration on dopamine release in the rat striatum has shown a pronounced difference between them. A repeated-measures analysis of variance revealed a significant effect of Treatments (Saline-treated group—Control, Acute Rotenone and Repeated Rotenone groups) (F 2,108 = 7.619, P < 0.00001). A post-hoc test indicated that repeated but not acute Rotenone administration produced a huge decrease in dopamine concentration (about 65–75% of Control group, P < 0.01) in extracellular area of the rat striatum (Fig. 6). Both (R)- and (S)-1MeTIQ enantiomers were able to antagonize rotenone-induced suppression of dopamine release as it is demonstrated in Fig. 7. A repeated-measures analysis of variance revealed a significant effect of Treatment (F 3,144 = 4.402, P < 0.026), Time (F 3,144 = 7.020, P < 0.00001) and interaction Time versus Treatment (F 3,144 = 2.835, P < 0.00001). A post-hoc Duncan’s test demonstrated that (R)-1MeTIQ enantiomer acted more strongly than (S)-1MeTIQ and not only reversed the Rotenone-produced depletion of the dopamine concentration to Control value but markedly increased dopamine in the extracellular area of the rat striatum (Fig. 7).
Microdialysis study: The comparison of an acute and repeated administration (during 3 consecutives days) of rotenone (3 mg/kg s.c.) on in vivo dopamine release in rat striatum. The basal value from the point “−60” to “0”. The arrow in the point “0” indicates the drug injections: Control (saline group); 1 × Rotenone (acute rotenone 3 mg/kg s.c.); 3 × Rotenone (a third injection of rotenone 3 mg/kg s.c.). The number of animals in a group n = 4. The data are expressed as means ± S.E.M. * P < 0.05, ** P < 0.01 versus Control value (one-way analyses of variance ANOVA followed by Duncan’s post-hoc test when appropriate)
Microdialysis study: The effect of (R)- and (S)-1MeTIQ enantiomers on the suppression of dopamine release in rat striatum induced by repeated (during 3 consecutives days) rotenone (3 mg/kg s.c.) administration. Arrow in the point “0” indicates the drug injections: Control (saline group); 3 × Rotenone (a third injection of rotenone 3 mg/kg s.c. + Saline i.p.); 3 × Rotenone + R-1MeTIQ (a third injection of rotenone 3 mg/kg s.c. + (R)-1MeTIQ 50 mg/kg i.p.); 3 × Rotenone + S-1MeTIQ (a third injection of rotenone 3 mg/kg s.c. + (S)-1MeTIQ 50 mg/kg i.p.). The number of animals in a group n = 4. The data are expressed as means ± S.E.M. * P < 0.05, ** P < 0.01 versus Control value; + P < 0.05, ++ P < 0.01 versus 3 × Rotenone-treated group (one-way analyses of variance ANOVA followed by Duncan’s post-hoc test when appropriate)
Discussion
It is currently believed that Parkinson`s disease is caused not only by genetic, but also, to a large extent, by environmental factors, of which herbicides may play an important, though not exclusive role (Gorell et al. 1998). Rotenone was described as a neurotoxic agent, inducing in experimental animals a parkinsonian-like syndrome (Betarbet et al. 2000). Since rotenone is widely used as a pesticide in agriculture and to protect household pets against insect parasites, its neurotoxic properties may pose a threat to farmers and pet owners. Two lines of reasoning support the role of rotenone. Firstly, both in hereditary and spontaneous Parkinson`s disease the activity of mitochondrial complex I is decreased, and rotenone is a potent inhibitor of this complex (Horgan et al. 1968; Gutman et al. 1970; Swerdlow et al. 1996). Secondly, oxidative damage is strongly implicated in the neurodegeneration of dopaminergic neurons (Lamensdorf et al. 2000) and, as shown by us previously, rotenone potently activates dopamine metabolism along the oxidative pathway (Antkiewicz-Michaluk and Vetulani 2001). The formerly published data have demonstrated that the endogenous substance, racemate 1MeTIQ, a mixture of two (R)- and (S)- enantiomers, protects animals against rotenone-induced mortality and neurodegeneration in the extrapyramidal structures produced by its peripheral and intracerebral injection (Antkiewicz-Michaluk et al. 2003, 2004). A part of the observed behavioral abnormalities may be explained by the action of rotenone on the dopaminergic system. The repeated but not acute rotenone administration (7 days), in a dose of 12 mg/kg s.c. was found to strongly increase dopamine metabolism, as measured by the increase in concentration of the intraneuronal dopamine metabolite, DOPAC, and the final metabolite, HVA, and interestingly, at the same time it depressed the concentration of the extracellular dopamine metabolite, 3-MT (Antkiewicz-Michaluk et al. 2003). Such neurochemical changes do not imply a direct neurotoxic action, but the shift in dopamine catabolism toward the oxidative pathway, generating free radicals, which may lead to neurodegeneration (Antkiewicz-Michaluk et al. 2001; Miller et al. 1996). These changes were effectively counteracted by the administration of racemate (R,S)-1MeTIQ before each dose of rotenone (Antkiewicz-Michaluk et al. 2003). In another model of neurotoxicity produced by malonate (Green and Greenmyre 1995; Zeevalk et al. 1995), a reversible inhibitor of the mitochondrial enzyme succinate dehydrogenase a close derivative of 1MeTIQ, 1,2,3,4-tetrahydroisoquinoline (TIQ) expressed also neuroprotective activity (Lorenc-Koci et al. 2005). A large body of evidence from in vivo and in vitro studies show that the damage of dopamine neurons due to rotenone- and malonate-induced impairment of energy metabolism is a consequence of mechanisms which involve the generation of free radicals, toxicity of dopamine, and secondary excitotoxicity (Antkiewicz-Michaluk et al. 2003, 2004; Beal et al. 1993; Ferger et al. 1999; Green and Greenmyre 1995; Green et al. 1993; Moy et al. 2000). Consistent with this, endogenous tetrahydroisoquinolines: TIQ and 1MeTIQ which display free radical scavenging properties as well as MAO inhibitory properties that attenuate dopamine catabolism and are partial NMDA receptor antagonists preventing the activation of this receptor, provide effective protection from these neurotoxins (Antkiewicz-Michaluk et al. 2006; Lorenc-Koci et al. 2005; Patsenka and Antkiewicz-Michaluk 2004).
In this article, we chose a four times lower dose of rotenone (3 mg/kg) than was used by us previously, which in this study did not affect tissue concentration of dopamine, its metabolism and the rate of oxidative pathway in the rat striatum. However, the concentration of 3-MT and the rate of dopamine O-methylation was significantly depressed after repeated administration of rotenone, and these effects of rotenone were completely antagonized by both stereospecific enantiomers of 1MeTIQ (Table 2). The question arises: whether dopaminergic neuron function evaluated by in vivo dopamine release would be affected in such experimental conditions? The results from our paper demonstrated that firstly, thrice rotenone administration in a low dose produced a considerable, significant impairment of the striatal dopamine release in vivo. Secondly, both (R)- and (S)- stereospecific enantiomers of 1MeTIQ, displayed a protective action against the rotenone-induced behavioral and specially neurochemical disturbances. However, it should be mention that rotenone in this investigated low dose depressed slightly but not significantly even after repated administration a basic locomotor activity in rats (Figs. 3a, b, 4a, b). In fact, it should be taken into account that basic locomotor activity of control group was low, and in such case it would be difficult for any treatment makes it significant lower (Wasik et al. 2009a, b). Generally, the results from behavioral and neurochemical in vivo microdialysis studies have shown that neuroprotection offered by 1MeTIQ is not stereospecific and, its enantiomers (R)- and (S)-1MeTIQ which are both present in the brain produced similar to racemate effect as shown previously (Antkiewicz-Michaluk et al. 2003, 2004) protect dopamine neurons against rotenone-induced impairment of its function in the rat brain. In addition, the in vivo microdialysis study demonstrated that (R)-enantiomer acted more strongly than (S)-1MeTIQ, and additionally, it not only prevented the rotenone-induced fall of extracellular dopamine level but even increased it above the control concentration.
On the other hand, the biochemical studies obtained from striatal tissue are in agreement with the previous published data (Antkiewicz-Michaluk et al. 2009; Wasik et al. 2009a, b) and clearly demonstrated that (R)- and (S)-1MeTIQ enantiomers differently affected the dopamine metabolism rate and its catabolic pathways in the striatum (Tables 1, 2). (R)-Enantiomer activated dopamine system and significantly increased all metabolic dopamine parameters: its metabolic rate and the concentration of dopamine and its metabolites. In contrast to (R)-enantiomer, (S)-1MeTIQ behaved as an MAO inhibitor in neurochemical studies, and decreased the dopamine metabolic rate as well as DOPAC and HVA levels but increased 3-MT, an extraneuronal metabolite of dopamine in the rat striatum. In addition, the data has shown that (S)-1MeTIQ behaved similarly to the racemate (R,S)-1MeTIQ however, its neurochemical effect was even stronger (Antkiewicz-Michaluk et al. 2001; Antkiewicz-Michaluk et al. 2004). Taken together, it looks that both enantiomers activated dopamine system: (R)-1MeTIQ by increasing dopamine metabolism, (S)-1MeTIQ by inhibition of dopamine catabolism. It is also important to mention that in contrast to DOPAC and HVA levels, both stereospecif enantiomers affected the concentration of dopamine and its extraneuronal metabolite, 3-MT in the same direction significantly increasing their levels. It is important to mention that pronounced increase of dopamine O-methylation along COMT-dependent catabolic pathway may offers neuroprotection in contrast to dopamine oxidation directly connected with free radical production (Antkiewicz-Michaluk et al. 2001; Miller et al. 1996). It was also demonstrated by other authors that increasing of 3-MT in the process of dopamine O-methylation has the function of protecting against oxidative stress (Miller et al. 1996). Our present data have shown that repeated treatment of rotenone significantly depressed both the rate of dopamine O-methylation as 3-MT concentration in rat striatum, and this effect was completely antagonized by both 1MeTIQ enantiomers (Table 2). In that light we suggest that (R)- and (S)- stereospecific enantiomers of 1MeTIQ may offer neuroprotection. Besides, 3-MT is considered to be the most reliable indicator of dopamine release into the synaptic cleft (Egan et al. 1991; Karoum et al. 1994), and its rise after (R)- and (S)-1MeTIQ administration, observed in this study, indicates the increase in dopamine release into the extraneuronal space. In fact, these ex vivo biochemical data are in a good agreement with in vivo microdialysis experiments which indicate that the administration of both enantiomers, however, in greater degree (S)-1MeTIQ produced an increase of extracellular dopamine concentration in the rat striatum (Fig. 5a, b). In addition, behavioral data (a locomotor activity) also confirm a slight stimulatory effect of (R)- and (S)-enantiomers on dopaminergic system. In control as well as in rotenone-treated rats, the administration of both stereo-enantiomers significantly increased the distance of walking (Figs. 3a, b, 4a, b). However, Abe et al. (2001) have demonstrated in C57BL/6 N mice that (R)-1MeTIQ and its racemate but not (S)-enantiomer plays a crucial role in protection against TIQ-induced parkinsonism. In our study, not only (R)-1MeTIQ but also (S)-enantiomer antagonized the behavioral and neurochemical effects of repeated rotenone administration in the rat. Such discrepancy concerning neuroprotective effects of stereo-isomers of 1MeTIQ between our study and Abe et al. (2001) results may be connected with distinct experimental protocols which differed at least in animal species (rat, mouse) and substances producing Parkinson`s disease-like symptoms (rotenone, TIQ). Anyhow, Alam and Schmidt (2002) demonstrated also that rotenone in a similar low dose 2.5 mg/kg but in contrast to us administered for a long period of time (2 months) caused dopamine depletion in the striatum and prefrontal cortex of the rat. In our hands, a low dose of rotenone administered only three times significantly depressed the concentration of the striatal 3-MT. As mention above, 3-MT is the most reliable biochemical indicator of dopamine release into the synaptic cleft (Egan et al. 1991), and its fall may suggest outset of irregularities in function of dopamine neurons. In fact, in vivo microdialysis studies presented in this article confirmed the above suggestion and demonstrated a pronounced decrease in dopamine release in the striatum after repeated but not acute rotenone administration (Fig. 7). These in vivo results have shown for the first time the initial, important signs of rotenone functional neurotoxicity leading to a fall of the striatal dopamine release. The demonstration in this article of inhibition of the striatal dopamine release by rotenone provides a unifying explanation for the motor deficits that can accompany rotenone exposure, even when nigrostriatal degeneration is minimal. The question arises as to what could be a molecular mechanism of action for that rotenone effect. As it was already well established, synaptic dopamine homeostasis is primarily controlled via two presynaptic regulatory mechanisms, dopamine D2 receptor and dopamine transporter (DAT). Dopamine D2/3 receptors directly mediate dopamine synthesis and release by being associated with DAT and regulate also DAT function, linking dopamine release and reuptake to a common mechanism (Campiani et al. 2003; Chen et al. 2009; Kebabian and Calne 1979; Le Foll et al. 2000; Shi 2009). Rotenone causes impairment of mitochondrial respiratory enzyme function and reactive oxygen species (ROS) generation but does not affect directly D2/3 dopamine receptors or DAT function (Bowton et al. 2010). However, in vitro studies focused on a real-time dopamine release monitoring with carbon–fiber microelectrodes in guinea pig striatal slices have shown that partial mitochondrial inhibition induced by a low, nanomolar concentrations of rotenone causes striatal dopamine release suppression via hydrogen peroxide (H2O2) generation and consequent activation of ATP-sensitive K + channels (Bao et al. 2005). In fact, KATP channels are important structures in the regulation of dopamine release in the striatum and dopaminergic neuron activity in the substantia nigra, and its stimulation inhibits dopamine release (Avshalumov and Rice 2003; Avshalumov et al. 2005). Moreover, KATP channel opening mediates the physiological responses of dopamine neurons to nanomolar concentrations of rotenone (Liss et al. 1999). It has been recently also reported that rotenone activates microglia, which then release superoxide (O2 −) through the action of NADPH oxidation enzyme, thus exacerbating dopamine neurotoxicity (Gao et al. 2002; Wang et al. 2005). Taken together, in vivo results of our study are in a good agreement with those presented above in vitro experiments and indicate that rotenone in a low concentration causes an appreciable decline in the striatal dopamine release which would further enhance dopamine-dependent motor deficits even in the absence of dopamine fall in the nigrostriatal pathway. In this study, in vivo microdialysis analysis has shown that the rotenone-induced suppression of the striatal dopamine release was completely reversed by a single injection of both stereo-enantiomers of 1MeTIQ: (R)- and (S)-1MeTIQ. As demonstrated in the earlier articles, the most evident neuroprotective action among tetrahydroisoquinolines was described for racemate, (R,S)-1MeTIQ (Tasaki et al. 1991; Antkiewicz-Michaluk 2004; Antkiewicz-Michaluk et al. 2003, 2006), the compound that shows an interesting pharmacological profile and possibly plays the role of a natural agent protecting the brain against Parkinson’s disease. (R,S)-1MeTIQ was demonstrated to act as an antidopaminergic agent in the functional studies (Antkiewicz-Michaluk et al. 2000a, b; Antkiewicz-Michaluk and Vetulani 2001), but in contrast to typical neuroleptics, it did not induce catalepsy in animals at all. Moreover, it inhibited apomorphine-induced hyperactivity at doses at which had no effect on spontaneous locomotor activity of rats (Antkiewicz-Michaluk et al. 2001). An additional finding, which may shed some light on the mechanism of action of racemate, is the observation that (R,S)-1MeTIQ displaces agonists (dopamine, apomorphine) but not antagonists (spiperon) of dopamine receptor from their binding sites (Antkiewicz-Michaluk et al. 2007). This suggests that the racemate, (R,S)-1MeTIQ has an affinity for the agonistic (active) conformation of dopamine receptors and expresses the effects which are characteristic for dopamine partial agonists. We also previously found for (R,S)-1MeTIQ, that its structure inhibited free radical generation in an abiotic system, and diminished the indices of glutamate-induced neurotoxicity (caspase-3 activity, lactate dehydrogenase release) in mouse embryonic primary cell cultures (Antkiewicz-Michaluk et al. 2006) what would be also specially important for a possible explanation of neuroprotective activity of its (R)- and (S)- enantiomers. The present biochemical data additionally have shown that (R)- and (S)- stereospecific enantiomers of 1MeTIQ in contrast to rotenone significantly increased dopamine O-methylation process and its product, 3-MT which has a pronounced protecting function against oxidative stress (Miller et al. 1996). In that light we suggest that neuroprotection may be offered by both stereospecific enantiomers of 1MeTIQ.
In conclusion, this article has shown in ex vivo and in vivo experiments for the first time the neurochemical signs of rotenone-induced impairment of striatal dopamine function. An appreciable decline similar to the rate of dopamine O-methylation and striatal dopamine release was observed even in the absence of dopamine fall in the nigrostriatal pathway. The results also clearly demonstrate that neuroprotective activity of 1MeTIQ is connected with both, (R)-and (S)-stereospecifc enantiomers which exhibit antagonism to rotenone-produced suppresion of dopamine O-methylation as well as dopamine release in rat striatum. In that light, we may suggest that 1MeTIQ will be useful as a drug with marked neuroprotective activity in the brain.
References
Abe K, Taguchi K, Wasai T, Ren J, Utsunomiya I, Shinohara T, Miyatake T, Sano T (2001) Stereoselective effect of (R)- and (S)-1-methyl-1,2,3,4-tetrahydroisoquinolines on mouse model of Parkinson`s disease. Brain Res Bull 56:55–60
Abe K, Saitoh T, Horiguchi Y, Utsunomiya I, Taguchi K (2005) Synthesis and neurotoxicity of tetrahydroisoquinoline derivatives for studying Parkinson’s disease. Biol Pharm Bull 28:1355–1362
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136:317–324
Antkiewicz-Michaluk L (2004) Neuroprotective effects of 1-methyl-1,2,3,4-tetrahydroisoquinoline: in vitro and in vivo studies in rats. Pol J Pharmacol 56(Suppl):179
Antkiewicz-Michaluk L, Vetulani J (2001) Tetrahydroisoquinolines as endogenous neurotoxins and neuroprotectants. Acta Neurobiol Exp 61:246
Antkiewicz-Michaluk L, Michaluk J, Romańska I, Papla I, Vetulani J (2000a) Antidopaminergic effects of 1,2,3,4-tetrahydroisoquinoline and salsolinol. J Neural Transm 107:1009–1019
Antkiewicz-Michaluk L, Romańska I, Papla I, Michaluk J, Bakalarz M, Vetulani J, Krygowska-Wajs A, Szczudlik A (2000b) Neurochemical changes induced by acute and chronic administration of 1,2,3,4-tetrahydroisoquinoline and salsolinol in dopaminergic structures of rat brain. Neuroscience 96:59–64
Antkiewicz-Michaluk L, Michaluk J, Mokrosz M, Romańska I, Lorenc-Koci E, Ohta S, Vetulani J (2001) Different action on dopamine catabolic pathways of two endogenous 1,2,3,4-tetrahydroisoquinolines with similar antidopaminergic properties. J Neurochem 78:100–108
Antkiewicz-Michaluk L, Karolewicz B, Romańska I, Michaluk J, Bojarski AJ, Vetulani J (2003) 1-Methyl-1,2,3,4-tetrahydroisoquinoline protects against rotenone-induced mortality and biochemical changes in rat brain. Eur J Pharmacol 466:263–269
Antkiewicz-Michaluk L, Wardas J, Michaluk J, Romańska I, Bojarski A, Vetulani J (2004) Protective effect of 1-methyl-1,2,3,4-tetrahydroisoquinoline against dopaminergic neurodegeneration in the extrapyramidal structures produced by intracerebral injection of rotenone. Int J Neuropsychopharmacol 7:155–163
Antkiewicz-Michaluk L, Filip M, Kostowski W, Patsenka A, Popik P, Przegaliński E, Wróbel M (2005) 1-Methyl-1,2,3,4-tetrahydroisoquinoline attenuates ethanol, cocaine and morphine addiction in behavioral models: neurochemical correlates. Acta Neurobiol Exp 65:301–321
Antkiewicz-Michaluk L, Łazarewicz JW, Patsenka A, Kajta M, Ziemińska E, Salińska E, Wąsik A, Gołembiowska K, Vetulani J (2006) The mechanism of 1,2,3,4-tetrahydroisoquinolines neuroprotection: the importance of free radicals scavenging properties and inhibition of glutamate-induced excitotoxicity. J Neurochem 97:846–856
Antkiewicz-Michaluk L, Filip M, Michaluk J, Romańska I, Przegaliński E, Vetulani J (2007) An endogenous neuroprotectant substance, 1-methyl-1,2,3,4-tetrahydroisoqunoline (1MeTIQ), prevents the behavioral and neurochemical effects of cocaine reinstatement in drug-dependent rats. J Neural Transm 114:307–317
Antkiewicz-Michaluk L, Romańska I, Boksa J, Bojarski A, Michaluk J (2009) Different neurochemical effects of (R)- and (S)- enantiomers of tetrahydroisoquinoline on dopamine metabolism in rat brain. Eur Neuropsychopharmacol 19(Suppl 3):S261
Avshalumov MV, Rice ME (2003) Activation of ATP-sensitive K+(K(ATP)) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc Natl Acad Sci USA 100:11729–11734
Avshalumov MV, Chen BT, Koos T, Tepper JM, Rice ME (2005) Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci 25:4222–4231
Bankiewicz KS, Oldfield EH, Chiueh CC, Doppman JL, Jacobowitz DM, Kopin IJ (1986) Hemiparkinsonism in monkeys after unilateral internal carotid artery infusion of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Life Sci 39:7–16
Bao L, Avshalumov MV, Rice ME (2005) Partial mitochondrial inhibition causes striatal dopamine release suppression and medium spiny neuron depolarization via H2O2 elevation, not ATP depletion. J Neurosci 25:10029–10040
Beal MF, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman BT (1993) Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem 61:1147–1150
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Bowton E, Sauders C, Erreger K, Sakrikar D, Mathies HJ, Sen N, Jessen T et al (2010) Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. J Neurosci 30:6048–6057
Campiani G, Butini S, Trotta F, Fattorusso C, Catalanotti B, Aiello F, Gemma S, Nacci V et al (2003) Synthesis and pharmacological evaluation of potent and highly selective D3 receptor ligands: inhibition of cocaine-seeking behavior and the role of dopamine D3/D2 receptors. J Med Chem 46:3822–3839
Chen PC, Lao CL, Chen JC (2009) The D(3) dopamine receptor inhibits dopamine release in PC-12/hD3 cells by autoreceptor signaling via PP-2B, CK1, and Cdk-5. J Neurochem 110:1180–1190
Egan MF, Karoum F, Wyatt RJ (1991) Effects of acute and chronic clozapine and haloperidol administration on 3-methoxytyramine accumulation in the rat prefrontal cortex, nucleus accumbens and striatum. Eur J Pharmacol 199:191–199
Ferger B, Eberhardt O, Teismann P, de Groote C, Schulz JB (1999) Malonate-induced generation of reactive oxygen species in rat striatum depends on dopamine release but not on NMDA receptor activation. J Neurochem 73:1329–1332
Filip M, Antkiewicz-Michaluk L, Zaniewska M, Frankowska M, Gołda A, Vetulani J, Przegaliński E (2007) Effects of 1-methyl-1,2,3,4-tetrahydroisoquinoline on the behavioral effects of cocaine in rats. J Physiol Pharmacol 58:625–639
Gao HM, Hong JS, Zhang W, Liu B (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 103:987–1041
Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ (1998) The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 50:1346–1350
Green JG, Greenmyre JT (1995) Characterization of the excitotoxic potential of the reversible succinate dehydrogenase inhibitor malonate. J Neurochem 64:430–436
Green JG, Porter RH, Eller RV, Greenmyre JT (1993) Inhibition of succinate dehydrogenase by malonic acid produces an “excitotoxic” lesion in rat striatum. J Neurochem 61:1151–1154
Greenamyre JT, Betarbet R, Sherer T, Panov A (2001) Parkinson`s disease, pesticides and mitochondrial dysfunction. Trends Neurosci 24:247–252
Gutman M, Singer TP, Beinert H, Casida JE (1970) Reaction sites of rotenone, piericidin A, and amytal in relation to the nonheme iron components of NADH dehydrogenase. Proc Natl Acad Sci USA 65:763–770
Heikkila RE, Nicklas WJ, Vyas I, Duvoisin RC (1985) Dopaminergic toxicity of rotenone and the 1-methyl-4-phenylpyridinium ion after their stereotaxic administration to rats: implication for the mechanism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Neurosci Lett 62:389–394
Horgan DJ, Singer TP, Casida JE (1968) Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. 13. Binding sites of rotenone, piericidin A, and amytal in the respiratory chain. J Biol Chem 243:834–843
Jenner P (2001) Parkinson’s disease, pesticides and mitochondrial dysfunction. Trends Neurosci 24:245–247
Karoum F, Chrapusta SJ, Egan MF (1994) 3-Methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in frontal cortex, nucleus accumbens and striatum by a simple two pool model. J Neurochem 63:972–978
Kebabian JW, Calne DB (1979) Multiple receptors for dopamine. Nature 277:93–96
Kohno M, Ohta S, Hirobe M (1986) Tetrahydroisoquinoline and 1-methyltetrahydro-isoquinoline as novel endogenous amines in rat brain. Biochem Biophys Res Com 140:448–454
Kotake Y, Tasaki Y, Makino Y, Ohta S, Hirobe M (1995) 1-Benzyl-1,2,3,4-tetrahydroisoquinoline as a parkinsonism-inducing agent: a novel endogenous amine in mouse brain and parkinsonian CSF. J Neurochem 65:2633–2638
Kotake Y, Yoshida M, Ogawa M, Tasaki Y, Hirobe M, Ohta S (1996) Chronic administration of 1-benzyl-1,2,3,4-tetrahydroisoquinoline, an endogenous amine in the brain, induces parkinsonism in a primate. Neurosci Lett 217:69–71
Lamensdorf I, Eisenhofer G, Harvey-White J, Hayakawa Y, Kirk K, Kopin IJ (2000) Metabolic stress in PC12 cells induces the formation of the endogenous dopaminergic neurotoxin, 3,4-dihydroxyphenylacetaldehyde. J Neurosci Res 60:552–558
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980
Le Foll B, Schwartz JC, Sokoloff P (2000) Dopamine D3 receptor agents as potential new medications for drug addiction. Eur Psych 15:140–146
Liss B, Bruns R, Roeper J (1999) Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. EMBO J 18:833–846
Lorenc-Koci E, Śmiałowska M, Antkiewicz-Michaluk L, Gołembiowska K, Bajkowska M, Wolfarth S (2000) Effect of acute and chronic administration of 1,2,3,4-tetrahydroisoquinoline on muscle tone, metabolism of dopamine in the striatum and tyrosine hydroxylase immunocytochemistry in the substantia nigra in rat. Neuroscience 95:1049–1059
Lorenc-Koci E, Antkiewicz-Michaluk L, Wardas J, Zapała M, Wierońska J (2004) Effect of 1,2,3,4-tetrahydroisoquinoline administration under conditions of CYP2D inhibition on dopamine metabolism, level of tyrosine hydroxylase protein and the binding of [3H]GBR 12, 935 to dopamine transporter in the rat nigrostriatal dopaminergic system. Brain Res 1009:67–81
Lorenc-Koci E, Gołembiowska K, Wardas J (2005) 1,2,3,4-Tetrahydroisoquinoline protects terminals of dopaminergic neurons in the striatum against the malonate-induced neurotoxicity. Brain Res 1051:145–154
Lotharius J, O’Malley KL (2000) The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J Biol Chem 275:38581–38588
Marey-Semper I, Gelman M, Levi-Strauss M (1993) The high sensitivity to rotenone of striatal dopamine uptake suggests the existence of a constitutive metabolic deficiency in dopaminergic neurons from the substantia nigra. Eur J Neurosci 5:1029–1034
Miller J, Selhub J, Joseph J (1996) Oxidative damage caused by free radicals produced during catecholamine autooxidation: protective effects of O-methylation and melatonin. Free Radic Biol Med 21:241–249
Moy LY, Zeevalk GD, Sonsalla PK (2000) Role for dopamine in malonate-induced damage in vivo in striatum an in vitro in mesencephalic cultures. J Neurochem 74:1656–1665
Nagatsu T (1997) Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci Res 29:99–111
Nakamura K, Bindokas VP, Marks JD, Wright DA, Frim DM, Miller RJ, Kang UJ (2000) The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: the role of mitochondrial complex I and reactive oxygen species revisited. Mol Pharmacol 58:271–278
Niwa T, Takeda N, Kaneda N, Hashizume Y, Nagatsu T (1987) Presence of tetrahydroisoquinoline and 2-methyl-tetrahydroisoquinoline in parkinsonian and normal human brains. Biochem Biophys Res Commun 144:1084–1089
Ohta S, Kohno M, Makino Y, Tachikawa O, Hirobe M (1987) Tetrahydroisoquinoline and 1-methyl-1,2,3,4-tetrahydroisoquinoline are present in the human brain: relation to Parkinson`s disease. Biomed Res 8:453–456
Patsenka A, Antkiewicz-Michaluk L (2004) Inhibition of rodent brain monoamine oxidase and tyrosine hydroxylase by endogenous compounds, 1,2,3,4-tetrahydroisoquinoline alkaloids. Pol J Pharmacol 56:727–734
Santiago M, Granero L, Machado A, Cano J (1995) Complex I inhibitor effect on the nigral and striatal release of dopamine in the presence and absence of nomifensine. Eur J Pharmacol 280:251–256
Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem 54:823–827
Shi WX (2009) Electrophysiological characteristics of dopamine neurons: a 35-year update. J Neural Transm 73:103–119
Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JPJ, Davis RE, Parker WDJ (1996) Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol 40:663–671
Tasaki Y, Makino Y, Ohta S, Hirobe M (1991) 1-Methyl-1,2,3,4-tetrahydroisoquinoline, decreasing in 1-methyl-4-phenyl-1,2,3,6-tetrahydropiridine-treated mouse, prevents parkinsonism-like behavior abnormalities. J Neurochem 57:1940–1943
Testa CM, Sherer TB, Greenamyre JT (2005) Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res 134:109–118
Thiffault C, Langston JW, Di Monte DA (2000) Increased striatal dopamine turnover following acute administration of rotenone to mice. Brain Res 885:283–288
Wang X, Chen S, Ma G, Ye M, Lu G (2005) Involvement of proinflammatory factors, apoptosis, caspase-3 activation and Ca2+ disturbances in microglia activation-mediated dopaminergic cell degeneration. Mech Ageing Dev 126:1241–1254
Wąsik A, Romańska I, Antkiewicz-Michaluk L (2007) The effect of an endogenous compound 1-methyl-1,2,3,4-tetrahydroisoquinoline on morphine-induced analgesia, dependenceand neurochemical changes in dopamine metabolism in rat brain structures. J Physiol Pharmacol 58:235–252
Wąsik A, Michaluk J, Romańska I, Bojarski A, Antkiewicz-Michaluk L (2009a) The comparison of effects of the endogenous enantiomer, (R)- with the racemate (R, S)-1-methyl-1,2,3,4-tetrahydroisoquinoline on dopamine metabolism in the brain structures and its in vivo release in rat striatum. Pharmacol Rep 61:366–367
Wąsik A, Romańska I, Antkiewicz-Michaluk L (2009b) 1-Benzyl-1,2,3,4-tetrahydroisoquinoline, an endogenous parkinsonism-inducing toxin, strongly potentiates MAO-dependent dopamine oxidation and impairs dopamine release: ex vivo and in vivo neurochemical studies. Neurotox Res 15:15–23
Wróbel M (2005) 1-Methyl-1,2,3,4-tetrahydroisoquinoline attenuates ethanol, cocaine and morphine addiction in behavioral models: neurochemical correlates. Acta Neurobiol Exp 65:(P3.01):321
Yamakawa T, Ohta S (1999) Biosynthesis of a parkinsonism-preventing substance, 1-methyl-1,2,3,4-tetrahydroisoquinoline, is inhibited by parkinsonism-inducing compounds in rat brain mitochondrial fraction. Neurosci Lett 259:157–160
Yamakawa T, Kotake Y, Fujitani M, Shintani H, Makino Y, Ohta S (1999) Regional distribution of parkinsonism-preventing endogenous tetrahydroisoquinoline derivatives and an endogenous parkinsonism-preventing substance-synthesizing enzyme in monkey brain. Neurosci Lett 276:68–70
Zeevalk GD, Derr-Yellin E, Nicklas WJ (1995) Relative vulnerability of dopamine and GABA neurons in mesencephalic culture to inhibition of succinate dehydrogenase by malonate and 3-nitropropionic acid and protection by NMDA receptor blockade. J Pharmacol Exp Ther 275:1124–1130
Acknowledgments
Thanks are due to Dr Jan Boksa (Department of Medicinal Chemistry, Institute of Pharmacology PAS, Krakow, Poland) for the synthesis of (R)- and (S)-1MeTIQ enantiomers. We gratefully acknowledge the technical assistance of Maria Kafel and Krzysztof Michalski. This study was supported by Polish Committee of Scientific Research, under grant No. N401 004836, and by the statutory funds of the Institute of Pharmacology, Polish Academy of Sciences, Krakow, Poland.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Antkiewicz-Michaluk, L., Wąsik, A., Romańska, I. et al. Both Stereoselective (R)- and (S)-1-Methyl-1,2,3,4-tetrahydroisoquinoline Enantiomers Protect Striatal Terminals Against Rotenone-Induced Suppression of Dopamine Release. Neurotox Res 20, 134–149 (2011). https://doi.org/10.1007/s12640-010-9228-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-010-9228-5