Abstract
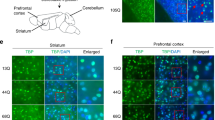
Spinocerebellar ataxia-1 (SCA1) is a late onset neurodegenerative disease caused by the expansion of a polyglutamine repeat within ataxin-1 protein. The toxic effects triggered by mutant ataxin-1 result in degeneration of the neurons in cerebellum, brain stem and spinocerebellar tracts. The targeted overexpression of mutant ataxin-1 in cerebellar Purkinje cells (PCs) of the SCA1 transgenic mice results in the formation of cytoplasmic vacuoles in PCs. These vacuoles appear early on before the onset of behavioral abnormalities. Interestingly, we found that vacoules contain S100B and vimentin proteins, which normally localize to neighboring Bergmann glia (BG). Further, immunohistochemical and specialized silver stain analysis revealed that vacuolar formation is associated with alterations in the morphology of dendritic spines of PCs. To gain insights into the mechanisms of vacuolar formation, we investigated if vacuoles in SCA1 PCs have an autophagic origin or are a consequence of some other event. We examined the expression levels (by Western blotting) of microtubule-associated protein light chain 3 (LC3)-I and LC3-II, and the degradation levels of p62 (a LC3 partner) in the cerebellar fractions prepared from pre-symptomatic SCA1 and age-matched wild-type mice. No p62 degradation was observed; however, LC3-II/(LC3-I + LC3-II) ratios were significantly altered in SCA1 mice indicating changes in the autophagic flux. In addition, LC3 localized to PC vacuoles. Further, we observed a co-localization of myo-inositol monophosphatase 1 (IMPA1) with S100B in PC vacuoles. IMPA1 is present in PC spines and has been implicated in autophagy. In vitro studies using purified IMPA1 and S100B demonstrated that S100B interacted with and activated IMPA1. Both apo and Ca2+-bound S100B were found to activate IMPA1, depending on substrate concentration. IMPA1 is regulated by another calcium-binding protein calbindin-D28k (CaB), since we reported earlier that the CaB levels are reduced in SCA1 PCs, the activation of IMPA1 by S100B may modulate CaB-dependent inositol signaling. This may cause BG–PC interface to degenerate resulting in vacuolar formation. In sum, these data indicate that vacuoles appearing early in SCA1 PCs could be developing through some unknown autophagic mechanism.










Similar content being viewed by others
References
Zoghbi HY, Orr HT (2000) Glutamine repeats and neurodegeneration. Annu Rev Neurosci 23:217–247
Koeppen AH (2005) The pathogenesis of spinocerebellar ataxia. Cerebellum 4:62–73
Matilla-Dueñas A, Goold R, Giunti P (2008) Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum 7:106–114
Orr HT, Chung M-Y, Banfi S, Kwiatkowski TJ Jr, Servadio A, Beaudet AL et al (1993) Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet 4:221–226
Banfi S, Servadio A, Chung MY, Kwiatkowski TJ Jr, McCall AE, Duvick LA et al (1994) Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet 7:513–520
Burright EN, Clark HB, Servadio A, Matilla T, Feddersen RM, Yunis WS et al (1995) SCA-1 transgenic mice: a model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 82:937–948
Skinner PJ, Vierra-Green CA, Clark HB, Zoghbi HY, Orr HT (2001) Altered trafficking of membrane proteins in Purkinje cells of SCA1 transgenic mice. Am J Pathol 159:905–913
Vig PJS, Lopez ME, Wei J, D’Souza DR, Subramony SH, Henegar J et al (2006) Glial S100B positive vacuoles in Purkinje cells: earliest morphological abnormality in SCA1 transgenic mice. J Neurol Sci [Turk] 23:166–174
Yamada K, Watanabe M (2002) Cytodifferentiation of Bergmann glia and its relationship with Purkinje cell. Anat Sci Int 2:94–108
Pakhotin P, Verkhratsky A (2005) Electrical synapses between Bergmann glial cells and Purkinje neurones in rat cerebellar slices. Mol Cell Neurosci 28:79–84
Slemmer JE, De Zeeuw CI, Weber JT (2005) Don't get too excited: mechanisms of glutamate-mediated Purkinje cell death. Prog Brain Res 148:367–390
Donato R (1991) Perspectives in S-100 protein biology. Cell Calcium 12:713–726
Donato R (2001) S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 33:637–668
Zimmer DB, Chaplin J, Baldwin A, Rast M (2005) S100-mediated signal transduction in the nervous system and neurological diseases. Cell Mol Biol 51:201–214
Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C et al (2006) Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat Neurosci 9:1302–1311
Donato R (1999) Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta 1450:191–231
Reeves RH, Yao J, Crowley MR, Buck S, Zhang X, Yarowsky P et al (1994) Astrocytosis and axonal proliferation in the hippocampus of S100b transgenic mice. Proc Natl Acad Sci U S A 91:5359–5363
Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H et al (2000) Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem 275:40096–40105
Rothermundt M, Peters M, Prehn JH, Arolt V (2003) S100B in brain damage and neurodegeneration. Microsc Res Tech 60:614–632
Winningham-Major F, Staecker JL, Barges SW, Coats S, VanElkik J (1989) Neurite extension and neuronal survival activities of recombinant S100β proteins that differ in the content and position of cysteine residues. J Cell Biol 109:3064–3071
Barger SW, VanEldik LJ, Mattson MP (1995) S100β protects hippocampal neurons from damage induced by glucose deprivation. Brain Res 677:167–170
Whitaker-Azmitia PM, Vingate M, Borella A, Gerlai R, Roder J, Azmitia EC (1997) Transgenic mice overexpressing the neurotrophic factor S-100β show neuronal cytoskeletal and behavioral signs of altered aging processes: implications for Alzheimer’s disease and Down’s syndrome. Brain Res 776:51–60
Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ et al (1989) Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A 86:7611–7615
Kato K, Suzuki F, Kurobe N, Okajima K, Ogasawara N, Nagaya M et al (1990) Enhancement of S-100β protein in blood of patients with Down’s syndrome. J Mol Neurosci 2:109–113
McClintock KA, Shaw GS (2000) A logical sequence search for S100B target proteins. Protein Sci 10:2043–2046
Wilder PT, Lin J, Bair CL, Charpentier TH, Yang D, Liriano M et al (2006) Recognition of the tumor suppressor protein p53 and other protein targets by the calcium-binding protein S100B. Biochim Biophys Acta 1763:1284–1297
Ohnishi T, Ohba H, Seo KC, Im J, Sato Y, Iwayama Y et al (2007) Spatial expression patterns and biochemical properties distinguish a second myo-inositol monophosphatase IMPA2 from IMPA1. J Biol Chem 282:637–646
Schmidt H, Schwaller B, Eilers J (2005) Calbindin D28k targets myo-inositol monophosphatase in spines and dendrites of cerebellar Purkinje neurons. Proc Natl Acad Sci U S A 120:5850–5855
Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M et al (2005) Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol 170:1101–1111
Sarkar S, Rubinsztein DC (2006) Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations. Autophagy 2:132–134
Tomomura M, Rice DS, Morgan JI, Yuzaki M (2001) Purification of Purkinje cells by fluorescence-activated cell sorting from transgenic mice that express green fluorescent protein. Eur J Neurosci 14:57–63
Vig PJS, Fratkin JD, Desaiah D, Currier RD, Subramony SH (1996) Decreased parvalbumin immunoreactivity in surviving Purkinje cells of patients with spinocerebellar ataxia-1. Neurology 47:249–253
Vig PJS, Subramony SH, Burright EN, Fratkin JD, McDaniel DO, Desaiah D et al (1998) Reduced immunoreactivity to calcium-binding proteins in Purkinje cells precedes onset of ataxia in spinocerebellar ataxia-1. Neurology 50:106–113
Vig PJS, Wei J, Shao Q, Hebert MD, Subramony SH, Sutton LT (2007) Role of tissue transglutaminase type 2 in calbindin-D28k interaction with ataxin-1. Neurosci Lett 420:53–57
Vig PJS, McDaniel DO, Subramony SH, Qin Z (1999) The effects of calbindin D-28k and parvalbumin antisense olivonucleotides on the survival of cultured Purkinje cells. Res Commun Mol Pathol Pharmacol 103:249–259
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3:542–545
Dahl D (1981) The vimentin-GFA protein transition in rat neuroglia cytoskeleton occurs at the time of myelination. J Neurosci Res 6:741–748
Shaw G, Osborn M, Weber K (1981) An immunofluorescence microscopical study of the neurofilament triplet proteins, vimentin and glial fibrillary acidic protein within the adult rat brain. Eur J Cell Biol 26:68–82
Galou M, Colucci-Guyon E, Ensergueix D, Ridet JL, Gimenez Y, Ribotta M et al (1996) Disrupted glial fibrillary acidic protein network in astrocytes from vimentin knockout mice. J Cell Biol 133:853–863
Colucci-Guyon E, Gimenez Y, Ribotta M, Maurice T, Babinet C, Privat A (1999) Cerebellar defect and impaired motor coordination in mice lacking vimentin. Glia 25:33–43
Ziegler DR, Innocente CE, Leal RB, Rodnight R, Goncalves CA (1998) The S100B protein inhibits phosphorylation of GFAP and vimentin in a cytoskeletal fraction from immature rat hippocampus. Neurochem Res 23:1259–1263
Sorci G, Agneletti AL, Donato R (2000) Effects of S100A1 and S100B on microtubule stability. An in vitro study using triton-cytoskeletons from astrocyte and myoblast cell lines. Neurosci 99:773–783
Bellamy TC (2006) Interactions between Purkinje neurones and Bergmann glia. Cerebellum 5:116–126
Lordkipanidze T, Dunaevsky A (2005) Purkinje cell dendrites grow in alignment with Bergmann glia. Glia 51:229–234
Gordon GA, Libby RT, Fu Y-H, Kinoshita Y, Huatag J, Possin DE et al (2002) Polyglutamine-expanded ataxin- promotes non-cell-autonomous Purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J Neurosci 22:4897–4905
Berggard T, Szczepankiewicz O, Thulin E, Linse S (2002) Myo-inositol monophosphatase is an activated target of calbindin D28k. J Biol Chem 277:41954–41959
Lowenstein DH, Miles MF, Hatam F, McCabe T (1991) Up regulation of calbindin-D28K mRNA in the rat hippocampus following focal stimulation of the perforant path. Neuron 6:627–633
Lowenstein DH, Gwinn RP, Seren MS, Simon RP, McIntosh TK (1994) Increased expression of mRNA encoding calbindin-D28K, the glucose-regulated proteins, or the 72 kDa heat-shock protein in three models of acute CNS injury. Brain Res Mol Brain Res 22:299–308
Vig PJS, Subramony SH, Qin Z, McDaniel DO, Fratkin J (2000) Relationship between ataxin-1 nuclear inclusions and Purkinje cell specific proteins in SCA-1 transgenic mice. J Neurol Sci 174:100–110
Acknowledgment
This work was supported by grants from the National Institute of Neurological Disorders and Stroke and National Ataxia Foundation, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vig, P.J.S., Shao, Q., Subramony, S.H. et al. Bergmann Glial S100B Activates Myo-inositol Monophosphatase 1 and Co-localizes to Purkinje Cell Vacuoles in SCA1 Transgenic Mice. Cerebellum 8, 231–244 (2009). https://doi.org/10.1007/s12311-009-0125-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-009-0125-5