Abstract
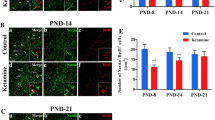
Mounting evidence suggests that prolonged exposure to general anesthesia (GA) during brain synaptogenesis damages the immature neurons and results in long-term neurocognitive impairments. Importantly, synaptogenesis relies on timely axon pruning to select axons that participate in active neural circuit formation. This process is in part dependent on proper homeostasis of neurotrophic factors, in particular brain-derived neurotrophic factor (BDNF). We set out to examine how GA may modulate axon maintenance and pruning and focused on the role of BDNF. We exposed post-natal day (PND)7 mice to ketamine using a well-established dosing regimen known to induce significant developmental neurotoxicity. We performed morphometric analyses of the infrapyramidal bundle (IPB) since IPB is known to undergo intense developmental modeling and as such is commonly used as a well-established model of in vivo pruning in rodents. When IPB remodeling was followed from PND10 until PND65, we noted a delay in axonal pruning in ketamine-treated animals when compared to controls; this impairment coincided with ketamine-induced downregulation in BDNF protein expression and maturation suggesting two conclusions: a surge in BDNF protein expression “signals” intense IPB pruning in control animals and ketamine-induced downregulation of BDNF synthesis and maturation could contribute to impaired IPB pruning. We conclude that the combined effects on BDNF homeostasis and impaired axon pruning may in part explain ketamine-induced impairment of neuronal circuitry formation.





Similar content being viewed by others
References
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23(3):876–882
Loepke AW, Istaphanous GK, McAuliffe JJ 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD et al (2009) The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg 108(1):90–104
Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA et al (2011) Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol 33(2):220–230
Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V (2008) Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol 18(2):198–210
Fredriksson A, Pontén E, Gordh T, Eriksson P (2007) Neonatal exposure to a combination of N-methyl-d-aspartate and gamma-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology 107(3):427–436
Viberg H, Pontén E, Eriksson P, Gordh T, Fredriksson A (2008) Neonatal ketamine exposure results in changes in biochemical substrates of neuronal growth and synaptogenesis, and alters adult behavior irreversibly. Toxicology 249(2–3):153–159
Kodama M, Satoh Y, Otsubo Y, Araki Y, Yonamine R, Masui K, Kazama T (2011) Neonatal desflurane exposure induces more robust neuroapoptosis than do isoflurane and sevoflurane and impairs working memory. Anesthesiology 115(5):979–991
Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW (2004) Axon branch removal at developing synapses by axosome shedding. Neuron 44:651–661
Luo L, O’Leary DD (2005) Axon retraction and degeneration in development and disease. Annu Rev Neurosci 28:127–156
Wilder RT, Flick RP, Sprung J et al (2009) Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 110:796–804
Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD (2008) Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci 11:649–658
Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V (2006) General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis 11:1603–1615
Pearn ML, Hu Y, Niesman IR, Patel HH, Drummond JC, Roth DM, Akassoglou K, Patel PM et al (2012) Propofol neurotoxicity is mediated by p75 neurotrophin receptor activation. Anesthesiology 116:352–361
Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW (2005) Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol 146(2):189–197
Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M (2003) Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell 113:285–299
Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V (2005) Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 135:815–827
Liu XB, Low LK, Jones EG, Cheng HJ (2005) Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J Neurosci 25:9124–9134
Lawrence JM, Black IB, Mytilineou C, Field PM, Raisman G (1979) Decentralization of the superior cervical ganglion in neonates impairs the development of the innervations of the iris. A quantitative ultrastructural study. Brain Res 168:13–19
Majdan M, Miller FD (1999) Neuronal life and death decisions: functional antagonism between the Trk and p75 neurotrophin receptors. Int J Dev Neurosci 17:153–161
Miller FD, Kaplan DR (2001) Neurotrophin signaling pathways regulating neuronal apoptosis. Cell Mol Life Sci 58:1045–1053
Mintz CD, Barrett KM, Smith SC, Benson DL, Harrison NL (2013) Anesthetics interfere with axon guidance in developing mouse neocortical neurons in vitro via a γ-aminobutyric acid type A receptor mechanism. Anesthesiology 118:825–833
Singh KK, Miller FD (2005) Activity regulates positive and negative neurotrophin-derived signals to determine axon competition. Neuron 45:837–845
Crusio WE, Schwegler H (2005) Learning spatial orientation tasks in the radial-maze and structural variation in the hippocampus in inbred mice. Behav Brain Funct 1(1):3–11
Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P (2004) Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res 153:367–376
Lu Y, Christian K, Lu B (2008) BDNF: a key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89(3):312–323
Thoenen H (1995) Neurotrophins and neuronal plasticity. Science 270:593–598
Lu B, Figurov A (1997) Role of neurotrophins in synapse development and plasticity. Rev Neurosci 8:1–12
Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B (2005) Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci 8:1069–1077
Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL et al (2005) Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 25:6156–6166
Acknowledgments
This study was supported by the grants R0144517 (NIH/NICHD), R0144517-S (NIH/NICHD), R01 GM118197 (NIH/NIGMS), R21 HD080281 (NIH/NICHD), John E. Fogarty Award 007423-128322 (NIH), and March of Dimes National Award, USA (to Vesna Jevtovic-Todorovic). Vesna Jevtovic-Todorovic was an Established Investigator of the American Heart Association. We thank Jonathan Park for his assistance with the morphometric analysis of the IPB pruning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
The experiments were approved by the Animal Use and Care Committees at the University of Colorado, the Office of Laboratory Animal Resources (OLAR), Aurora, Colorado and the Animal Use and Care Committees of the University of Virginia, Charlottesville, Virginia. The experiments were done in accordance with the Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Rights and permissions
About this article
Cite this article
Obradovic, A.L., Atluri, N., Dalla Massara, L. et al. Early Exposure to Ketamine Impairs Axonal Pruning in Developing Mouse Hippocampus. Mol Neurobiol 55, 164–172 (2018). https://doi.org/10.1007/s12035-017-0730-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0730-0