Abstract
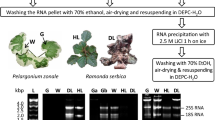
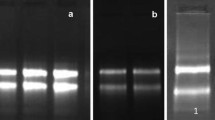
RNA isolation is a prerequisite for the study of the molecular mechanisms of stress tolerance in the desert plant Reaumuria soongorica, an extreme xeric semi-shrub. However, R. soongorica that contains high levels of secondary metabolites that co-precipitate with RNA, making RNA isolation difficult. Here the authors propose a new protocol suitable for isolating high-quality RNA from the leaves of R. soongorica. Based on a CTAB method described by Liu et al., the protocol has been improved as follows: the samples were ground with PVPP to effectively inhibit the oxidation of phenolics, contaminating DNA was removed with DNase I, and NaAc was used along with ethanol for precipitation to enhance the RNA yield and shorten the precipitation time. Gel electrophoresis and spectrophotometric analysis indicated that this isolation method provides RNA with no DNA contamination. Moreover, the yield (183.79 ± 40.36 μg/g) and quality were superior to those using the method of Liu et al., which yields RNA with significant DNA contamination at 126.30 ± 29.43 μg/g. Gene amplification showed that the RNA obtained using this protocol is suitable for use in downstream molecular procedures. This method was found to work equally well for isolating RNA from other desert plants. Thus, it is likely to be widely applicable.


Similar content being viewed by others
Abbreviations
- CTAB:
-
Cetyl trimethyl ammonium bromide
- DEPC:
-
Diethyl pyrocarbonate
- EDTA:
-
Ethylenediaminetetraacetic acid
- LiCl:
-
Lithium chloride
- NaAc:
-
Sodium acetate
- PCI:
-
Phenol: chloroform: isoamylal alcohol
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- PVPP:
-
Polyvinyl polypyrrolidone
- SDS:
-
Sodium dodecyl sulfate
References
Liu, W., Wang, B., Duan, C., & Li, B. (2005). A method for isolating functional RNA callus of Dendrobium candidum contented rich polysaccharides. Colloids and Surfaces B: Biointerfaces, 4, 259–262.
Wang, X. C., Tain, W. M., & Li, Y. X. (2008). Development of an efficient protocol of RNA isolation from recalcitrant tree tissues. Molecular Biotechnology, 38, 57–64.
Nap Bekesiovai, J. P., & Mlynarova, L. (1999). Plant Molecular Biology Reporter, 17, 269–277.
Gehrig, H. H., Winter, K., Cushman, J., Borland, A., & Taybi, T. (2000). An improved RNA isolation method for succulent plant species rich in polyphenols and polysaccharides. Plant Molecular Biology Reports, 18, 369–376.
Geuna, F., Hartings, H., & Scienza, A. (1998). A new method for rapid extraction of high quality RNA from recalcitrant tissues of grapevines. Plant Molecular Biology Reporter, 16, 61–67.
Wadsworth, G. J., Redinbaugh, M. G., & Scandalios, J. G. (1988). A procedure for the small scale isolation of plant RNA suitable for RNA blot analysis. Analytical Biochemistry, 172, 279–283.
Vicient, C. M., & Delseny, M. (1999). Isolation of total RNA from Arabidopsis thaliana seeds. Analytical Biochemistry, 268, 412–413.
Chomczynski, P., & Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Analytical Biochemistry, 162, 156–159.
Li, B., Wang, B. C., Tang, K., Liang, Y. L., Wang, J., & Wei, J. (2006). A simple and convenient approach for isolating RNA from highly viscous plant tissue rich in polysaccharides. Colloids and Surfaces B: Biointerfaces, 49, 101–105.
Kansal, R., Kuhar, K., Verma, I., Gupta, R. N., Gupta, V. K., & Koundal, K. R. (2008). Improved and convenient method of RNA isolation from polyphenols and polysaccharide rich plant tissues. Indian Journal of Experimental Biology, 46, 842–845.
Wang, L. M., & Stegemann, J. P. (2010). Extraction of high quality RNA from polysaccharide matrices using cetyltrimethylammonium bromide. Biomaterials, 31, 1612–1618.
Loomis, W. D. (1974). Overcoming problems of phenolic and quinines in the isolation of plant enzymes and organelles. Methods in Enzymology, 31, 528–545.
Birtic, S., & Kranner, I. (2006). Isolation of high-quality RNA from polyphenol-, polysaccharide- and lipid-rich seeds. Phytochemical Analysis, 17, 144–148.
Manickavelu, A., Kambara, K., Mishina, K., & Koba, T. (2007). An efficient method for purifying high quality RNA from wheat pistils. Colloids and Surfaces B: Biointerfaces, 54, 254–258.
Liu, J. Q., Qiu, M. X., Pu, J. C., & Lu, Z. M. (1982). The typical extreme xerophyte Reaumuria soongorica in the desert of China. Acta Botanica Sinica, 24, 485–488.
Liu, Y. B., Zhang, T. G., An, L. Z., & Wang, G. (2006). Isolation of RNA using modified method of CTAB from dehydrated and nondehydrated Reaumuria soongorica tissues. Journal of Desert Research, 26, 600–603.
Liu, Y. B., Zhao, X., & Tan, H. J. (2008). Induction and proliferation of callus in desert plant Reaumuria soongorica. Journal of Desert Research, 28, 254–258.
Asif, M. H., Dhawan, P., & Nath, P. (2000). A simple procedure for the isolation of high quality RNA from ripening banana fruit. Plant Molecular Biology Reporter, 18, 109–115.
Logemann, J., Schell, J., & Willmitzer, L. (1987). Improved method for the isolation of RNA from plant tissues. Analytical Biochemistry, 163, 16–20.
Manning, K. (1990). Isolation of nucleic acids from plants by differential solvent precipitation. Analytical Biochemistry, 195, 45–50.
Li, R., Mock, R., Huang, Q., Abad, J., Hartung, J., & Kinard, G. (2008). A reliable and inexpensive method of nucleic acid extraction for the PCR-based detection of diverse plant pathogens. Journal of Virological Methods, 154, 48–55.
Azevedo, H., Lino-Neto, T., & Tavares, R. M. (2003). An improved method for high-quality RNA isolation from needles of adult maritime pine trees. Plant Molecular Biology Reporter, 21, 333–338.
Jaakola, L., Pirttila, A. M., Halonen, M., & Hohtola, A. (2001). Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Molecular Biotechnology, 19, 201–203.
Porebski, S., Bailey, L., & Baum, B. (1997). Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Molecular Biology Reporter, 15, 8–15.
Chang, S., Puryear, J., & Cairney, J. (1993). A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter, 11, 113–116.
Fang, G., Hammar, S., & Grumet, R. (1992). A quick and inexpensive method for removing polysaccharides from plant genomic DNA. Biofeedback, 13, 52–54.
Maliyakal, E. J. (1992). An efficient method for isolation of RNA and DNA from plants containing polyphenolics. Nucleic Acids Research, 20, 2381.
Salzman, R. A., Fujita, T., Salzman, K. Z., Hasegawa, P. M., & Bressan, R. A. (1999). An improved RNA isolation method for plant tissues containing high levels of phenolic compounds or carbohydrates. Plant Molecular Biology Reporter, 17, 11–17.
Venkatachalam, P., Thanseem, I., & Thulaseedharan, A. (1999). A rapid and efficient method for isolation of RNA from bark tissues of Hevea brasiliensis. Current Science, 77, 635–637.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: a laboratory manual (2nd ed.). New York: Cold Spring Harbor Laboratory Press.
Gareth, P., Asuncion, L. L., Marta, V., & Ester, S. (2006). Simple and rapid RNA extraction from freeze-dried tissue of brown algae and seagrasses. European Journal of Phycology, 41, 97–104.
Birnboim, H. C. (1992). Extraction of high molecular weight RNA and DNA from cultured mammalian cells. Methods Enzymology, 216, 154–160.
Chen, G. Y. J., Jin, S., & Goodwin, P. H. (2000). An improved method for the isolation of total RNA from Malva pusilla tissues infected with Colletotrichum gloeosporioides. Journal of Phytopathology, 148, 57–60.
Dellacorte, C. (1994). Isolation of nucleic acids from the sea anemone Condylactis gigantea (Cnidaria: anthozoa). Tissue and Cell, 26, 613–619.
Mannan, M. A., Sharma, S., & Ganesan, K. (2009). Total RNA isolation from recalcitrant yeast cells. Analytical Biochemistry, 389, 77–79.
Acknowledgments
This study was supported by the National Key Technologies R&D Program of the 11th five-year plan (2007BAD46B) and the National Natural Science Foundation of China (Grant no. 40901019/D010102).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Xiao, H., Chen, G. et al. Isolation of High-Quality RNA from Reaumuria soongorica, a Desert Plant Rich in Secondary Metabolites. Mol Biotechnol 48, 165–172 (2011). https://doi.org/10.1007/s12033-010-9357-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-010-9357-3