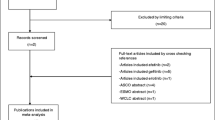
Abstract
The meta-analysis evaluated the efficacy and safety of chemotherapy or tyrosine kinase inhibitors combined with bevacizumab versus chemotherapy or tyrosine kinase inhibitors alone in the treatment of non-small cell lung cancer (NSCLC). The PubMed/MEDLINE, Ovid, Web of Science, CNKI, and the Cochrane Library database were searched for eligible randomized controlled trials comparing the combination of chemotherapy or epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) with bevacizumab to chemotherapy or EGFR-TKI alone. Main outcome measures were overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and adverse effects. The pooled data were analyzed by STATA 12.0 and expressed as hazard ratio (HR) or risk ratio (RR), with their corresponding 95 % confidence intervals (95 % CI). Nine eligible trials comprising 3,547 patients (1,779 for bevacizumab and 1,768 for controls) were included in the study. Chemotherapy or TKIs in combination with bevacizumab significantly prolonged PFS (HRpfs 0.72, 95 % CIpfs 0.66–0.79, P pfs < 0.001) and OS (HRos 0.90, 95 % CIos 0.82–0.99, P os = 0.029) as first-line treatment for NSCLC compared with chemotherapy or TKIs alone. Bevacizumab combination regimens significantly prolonged PFS (HR 0.62, 95 % CI 0.52–0.74, P < 0.001) as second-line treatment; however, no benefit regarding OS was observed with the addition of bevacizumab (HR 0.94, 95 % CI 0.78–1.12, P = 0.479). The bevacizumab group showed increased ORR in both first- and second-line treatments. The high-dose bevacizumab subgroup in combination with chemotherapy showed a statistically significant improvement in OS, PFS, and ORR (HRos 0.89, 95 % CIos 0.80–0.99, P os 0.037; HRpfs 0.71, 95 % CIpfs 0.64–0.79, P pfs < 0.01, RRorr 1.85, 95 % CIorr 1.59–2.15, P orr < 0.001, respectively); however, the low-dose bevacizumab subgroup did not show enhanced OS (HRos 0.91, 95 % CIos 0.77–1.07, P os = 0.263), and a moderate improvement of PFS and ORR (HRpfs 0.85, 95 % CIpfs 0.72–1.00, P pfs = 0.049; RRorr 1.60, 95 % CIorr 1.28–2.0, P orr < 0.001). Erlotinib in combination with bevacizumab significantly prolonged PFS (HR 0.60, P < 0.001, 95 % CI 0.51–0.71) and increased ORR (RR 1.21, 95 % CI 0.98–1.49, P = 0.067) compared with erlotinib alone. A higher incidence of grade ≥3 adverse events such as proteinuria, hypertension, and hemorrhage was observed in the bevacizumab combination group than in the control group without bevacizumab (P all < 0.05). The addition of bevacizumab to chemotherapy or erlotinib can significantly improve PFS and ORR both in first- and second-line treatments of advanced NSCLC, with an acceptable risk of bleeding events, hypertension, proteinuria, and rash. Combination therapy with bevacizumab and chemotherapy is beneficial regarding OS; however, whether bevacizumab plus erlotinib can prolong OS need further validation.






Similar content being viewed by others
References
NSCLC Meta-Analyses Collaborative Group. Chemotherapy in addition to supportive care improves survival in advanced non-small-cell lung cancer: a systematic review and meta-analysis of individual patient data from 16 randomized controlled trials. J Clin Oncol. 2008;26(28):4617–25. doi:10.1200/JCO.2008.17.7162.
Peters S, Adjei AA, Gridelli C, Reck M, Kerr K, Felip E, ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–64.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50.
Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–34. doi:10.1200/JCO.2007.14.5466.
Savas P, Hughes B, Solomon B. Targeted therapy in lung cancer: IPASS and beyond, keeping abreast of the explosion of targeted therapies for lung cancer. J Thorac Dis. 2013;5(Suppl 5):S579–92. doi:10.3978/j.issn.2072-1439.2013.08.52.
Belani CP, Goss G, Blumenschein G Jr. Recent clinical developments and rationale for combining targeted agents in non-small cell lung cancer (NSCLC). Cancer Treat Rev. 2012;38(3):173–84. doi:10.1016/j.ctrv.2011.05.009.
Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, Vlahovic G, Soh CH, O’Connor P, Hainsworth J. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. 2011;377(9780):1846–54. doi:10.1016/S0140-6736(11)60545-X.
Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, Nagase S, Okamoto I, Yamanaka T, Tajima K, Harada R, Fukuoka M, Yamamoto N. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15(11):1236–44. doi:10.1016/S1470-2045(14)70381-X.
Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25(30):4743–50.
Das M, Wakelee H. Targeting VEGF in lung cancer. Expert Opin Ther Targets. 2012;16(4):395–406. doi:10.1517/14728222.2012.669752.
NCCN guidelines version 2.2014 panel members non-small cell lung cancer
Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12(6):713–8.
Falchook GS, Naing A, Hong DS, Zinner R, Fu S, Piha-Paul SA, Tsimberidou AM, Morgan-Linnell SK, Jiang Y, Bastida C, Wheler JJ, Kurzrock R. Dual EGFR inhibition in combination with anti-VEGF treatment: a phase I clinical trial in non-small cell lung cancer. Oncotarget. 2013;4(1):118–27.
Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, Blumenschein G Jr, Lee JJ, Liu DD, Truong MT, Hong WK, Tran H, Tsao A, Xie D, Ramies DA, Mass R, Seshagiri S, Eberhard DA, Kelley SK, Sandler A. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23(11):2544–55.
Tortora G, Ciardiello F, Gasparini G. Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol. 2008;5(9):521–30. doi:10.1038/ncponc1161.
West HJ, Moon J, Hirsch FR, Mack PC, Wozniak AJ, Lau D, Fehrenbacher L, Bury MJ, Redman MW, Gandara DR. SWOG S0635 and S0636: Phase II trials in advanced-stage NSCLC of erlotinib (OSI-774) and bevacizumab in bronchioloalveolar carcinoma (BAC) and adenocarcinoma with BAC features (adenoBAC), and in never-smokers with primary NSCLC adenocarcinoma (adenoCa). J Clin Oncol. 2012;30(Suppl):abstr 7517.
Akerley W, Boucher K, Rich N, Egbert L, Harker G, Bylund J, Van Duren T, Reddy C. A phase II study of bevacizumab and erlotinib as initial treatment for metastatic non-squamous, non-small cell lung cancer with serum proteomic evaluation. Lung Cancer. 2013;79(3):307–11. doi:10.1016/j.lungcan.2012.12.005.
Zhang T, Yuan S, Wang Z, Zhang Q, Zhao P, Shan L. Bevacizumab combined with chemotherapy for advanced non-small cell lung cancer: a meta-analysis. Zhongguo Fei Ai Za Zhi. 2013;16(2):82–90. doi:10.3779/j.issn.1009-3419.2013.02.05.
Soria JC, Mauguen A, Reck M, Sandler AB, Saijo N, Johnson DH, Burcoveanu D, Fukuoka M, Besse B, Pignon JP. Meta-analysis of bevacizumab in advanced NSCLC collaborative group. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24(1):20–30. doi:10.1093/annonc/mds590.
Botrel TE, Clark O, Clark L, Paladini L, Faleiros E, Pegoretti B. Efficacy of bevacizumab (Bev) plus chemotherapy (CT) compared to CT alone in previously untreated locally advanced or metastatic non-small cell lung cancer (NSCLC): systematic review and meta-analysis. Lung Cancer. 2011;74(1):89–97. doi:10.1016/j.lungcan.2011.01.028.
Lima AB, Macedo LT, Sasse AD. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: a systematic review and meta-analysis. PLoS One. 2011;6(8):e22681. doi:10.1371/journal.pone.0022681.
Huang H, Zheng Y, Zhu J, Zhang J, Chen H, Chen X. An updated meta-analysis of fatal adverse events caused by bevacizumab therapy in cancer patients. PLoS One. 2014;9(3):e89960. doi:10.1371/journal.pone.0089960.
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–91.
Soria JC, Márk Z, Zatloukal P, Szima B, Albert I, Juhász E, Pujol JL, Kozielski J, Baker N, Smethurst D, Hei YJ, Ashkenazi A, Stern H, Amler L, Pan Y, Blackhall F. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29(33):4442–51. doi:10.1200/JCO.2011.37.2623.
Niho S, Kunitoh H, Nokihara H, Horai T, Ichinose Y, Hida T, Yamamoto N, Kawahara M, Shinkai T, Nakagawa K, Matsui K, Negoro S, Yokoyama A, Kudoh S, Kiura K, Mori K, Okamoto H, Sakai H, Takeda K, Yokota S, Saijo N, Fukuoka M, JO19907 Study Group. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer. 2012;76(3):362–7. doi:10.1016/j.lungcan.2011.12.005.
Boutsikou E, Kontakiotis T, Zarogoulidis P, Darwiche K, Eleptheriadou E, Porpodis K, Galaktidou G, Sakkas L, Hohenforst-Schmidt W, Tsakiridis K, Karaiskos T, Zarogoulidis K. Docetaxel-carboplatin in combination with erlotinib and/or bevacizumab in patients with non-small cell lung cancer. Onco Targets Ther. 2013;6:125–34. doi:10.2147/OTT.S42245.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, L., Ma, JT., Zhang, SL. et al. Efficacy and safety of chemotherapy or tyrosine kinase inhibitors combined with bevacizumab versus chemotherapy or tyrosine kinase inhibitors alone in the treatment of non-small cell lung cancer: a systematic review and meta-analysis. Med Oncol 32, 34 (2015). https://doi.org/10.1007/s12032-014-0473-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-014-0473-y