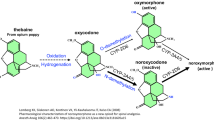
Abstract
Purpose of Review
This study and literature review were carried out to investigate whether oxycodone is the most addictive prescription opioid.
Recent Findings
This was a cross-sectional survey from a pain management practice in south-central Alaska and review of the literature involving 86 patients diagnosed with opioid dependence/opioid use disorder from 2013 to 2018. Patients were given a list of prescription opioids and asked to identify the one (1) most desirable to themselves, (2) most desirable among drug-using associates or community, and (3) they deemed most addictive. Patients with a history of heroin use were asked which, if any, served as their gateway drug to heroin. The literature was reviewed using a PubMed search for articles containing the words “oxycodone” and “abuse,” “addiction,” “dependence,” “disorder,” and “euphoria.” Oxycodone was ranked most highly in all four questions (n = 50, 60.2%; n = 46, 75.4%; n = 38, 60.2%; n = 14, 77.8%, respectively) by a wide margin.
Summary
Numerous observational studies performed over the past few decades have demonstrated the supreme “likability” and abuse and dependence liability/addictiveness of oxycodone, with more recent mechanistic studies illuminating biological underpinnings including markedly increased active transport across the blood-brain barrier, increased phasic dopaminergism in the ventral tegmental area, nucleus accumbens and related striatal reward centers, and possibly increased kappa opioid receptor-mediated withdrawal dysphoria. Oxycodone possesses pharmacologic qualities that render it disproportionately liable to abuse and addiction and the risks of any long-term prescription outweigh the benefits.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Halpern IM, Bonica JJ. Analgesics. In: Modell W, editor. Drugs of Choice 1976–1977. St. Louis: Mosby; 1976. p. 213.
Sneader W. Drug discovery: a history. Hoboken: Wiley; 2005.
McAnally H. Opioid dependence: a clinical and epidemiologic approach. Cham: Springer International Publishing; 2017.
Jones CM, Muhuri PK, Lurie PG. Trends in the nonmedical use of OxyContin, United States, 2006 to 2013. Clin J Pain. 2017;33(5):452–61. https://doi.org/10.1097/AJP.0000000000000426.
Dyer O. Kentucky seeks $1bn from Purdue pharma for misrepresenting addictive potential of oxycodone. BMJ. 2014;349:g6605. https://doi.org/10.1136/bmj.g6605.
Rosenberg D. Kentucky’s pain. Newsweek. 2004;144(12):44–5.
Schaefer CP, Tome ME, Davis TP. The opioid epidemic: a central role for the blood brain barrier in opioid analgesia and abuse. Fluids Barriers CNS. 2017;14(1):32. https://doi.org/10.1186/s12987-017-0080-3.
Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther. 2006;79:461–79.
• Villesen HH, Foster DJ, Upton RN, Somogyi AA, Martinez A, Grant C. Cerebral kinetics of oxycodone in conscious sheep. J Pharm Sci. 2006, 95:1666–76 (excellent review of cerebral kinetics of oxycodone).
Boström E, Simonsson US, Hammarlund-Udenaes M. In vivo blood-brain barrier transport of oxycodone in the rat: indications for active influx and implications for pharmacokinetics/pharmacodynamics. Drug Metab Dispos. 2006;34:1624–31.
• Boström E, Hammarlund-Udenaes M, Simonsson US. Blood-brain barrier transport helps to explain discrepancies in in vivo potency between oxycodone and morphine. Anesthesiology. 2008;108:495–505 (excellent discussion of blood-brain barrier transport related to oxycodone and morphine).
Okura T, Hattori A, Takano Y, Sato T, Hammarlund-Udenaes M, Terasaki T, et al. Involvement of the pyrilamine transporter, a putative organic cation transporter, in blood-brain barrier transport of oxycodone. Drug Metab Dispos. 2008;36:2005–13.
Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, et al. Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br J Pharmacol. 2010;160:919–30.
Stamer UM, Zhang L, Book M, Lehmann LE, Stuber F, Musshoff F. CYP2D6 genotype dependent oxycodone metabolism in postoperative patients. PLoS One. 2013;8(3):e60239. https://doi.org/10.1371/journal.pone.0060239.
Klimas R, Witticke D, El Fallah S, Mikus G. Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration. Expert Opin Drug Metab Toxicol. 2013;9:517–28.
Schoedel KA, McMorn S, Chakraborty B, Zerbe K, Sellers EM. Reduced cognitive and psychomotor impairment with extended-release oxymorphone versus controlled-release oxycodone. Pain Physician. 2010;13:561–73.
Schoedel KA, McMorn S, Chakraborty B, Potts SL, Zerbe K, Sellers EM. Positive and negative subjective effects of extended-release oxymorphone versus controlled-release oxycodone in recreational opioid users. J Opioid Manag. 2011;7:179–92.
Zwisler ST, Enggaard TP, Mikkelsen S, Brosen K, Sindrup SH. Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol Scand. 2010;54:232–40.
Söderberg Löfdal KC, Andersson ML, Gustafsson LL. Cytochrome P450-mediated changes in oxycodone pharmacokinetics/pharmacodynamics and their clinical implications. Drugs. 2013;73:533–43.
Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacol. 2003;170:242–54.
Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacol. 2008;196:105–16.
Zacny JP, Gutierrez S. Subjective, psychomotor, and physiological effects profile of hydrocodone/acetaminophen and oxycodone/acetaminophen combination products. Pain Med. 2008;9:433–43.
Zacny JP, Gutierrez S. Within-subject comparison of the psychopharmacological profiles of oral hydrocodone and oxycodone combination products in non-drug-abusing volunteers. Drug Alcohol Depend. 2009;101:107–14.
Comer SD, Sullivan MA, Whittington RA, Vosburg SK, Kowalczyk WJ. Abuse liability of prescription opioids compared to heroin in morphine-maintained heroin abusers. Neuropsychopharmacology. 2008;33:1179–91.
Katz N, Fernandez K, Chang A, Benoit C, Butler SF. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008;24:528–35.
•• Cicero TJ, Ellis MS, Paradis A, Ortbal Z. Determinants of fentanyl and other potent μ opioid agonist misuse in opioid-dependent individuals. Pharmacoepidemiol Drug Saf. 2010;19:1057–63 (excellent review of the determinants of potent μ opioid agonist misuse in opioid-dependent individuals).
• Cicero TJ, Ellis MS, Surratt HL, Kurtz SP. Factors influencing the selection of hydrocodone and oxycodone as primary opioids in substance abusers seeking treatment in the United States. Pain. 2013;154:2639–48 (excellent review of the factors influencing selection of primary opioids in substance abusers).
Osgood ED, Eaton TA, Trudeau JJ, Katz NP. A brief survey to characterize oxycodone abuse patterns in adolescents enrolled in two substance abuse recovery high schools. Am J Drug Alcohol Abuse. 2012;38:166–70.
Vosburg SK, Eaton TA, Sokolowska M, Osgood ED, Ashworth JB, Trudeau JJ, et al. Prescription opioid abuse, prescription opioid addiction, and heroin abuse among adolescents in a recovery high school: a pilot study. J Child Adolesc Subst Abuse. 2015;25:105–12.
Setnik B, Roland CL, Goli V, Pixton GC, Levy-Cooperman N, Smith I, et al. Self-reports of prescription opioid abuse and diversion among recreational opioid users in a Canadian and a United States city. J Opioid Manag. 2015;11:463–73.
Hays LR. A profile of OxyContin addiction. J Addict Dis. 2004;23:1–9.
Daniulaityte R, Carlson RG, Kenne DR. Initiation to pharmaceutical opioids and patterns of misuse: preliminary qualitative findings obtained by the Ohio substance abuse monitoring network. J Drug Issues. 2006;36:787–804.
Kalso E, Vainio A. Morphine and oxycodone hydrochloride in the management of cancer pain. Clin Pharmacol Ther. 1990;47:639–46.
Heiskanen T, Kalso E. Controlled-release oxycodone and morphine in cancer related pain. Pain. 1997;73:37–45.
Mucci-LoRusso P, Berman BS, Silberstein PT, Citron ML, Bressler L, Weinstein SM, et al. Controlled-release oxycodone compared with controlled-release morphine in the treatment of cancer pain: a randomized, double-blind, parallel-group study. Eur J Pain. 1998;2:239–49.
• Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, et al. Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. Eur J Neurosci. 2014;40:3041–54 (excellent investigation demonstrating dramatic difference between morphine and oxycodone dopaminergism).
Wanat MJ, Willuhn I, Clark JJ, Phillips PE. Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2:195–213.
Covey DP, Roitman MF, Garris PA. Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci. 2014;37:200–10.
Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–8.
Frank ST, Krumm B, Spanagel R. Cocaine-induced dopamine overflow within the nucleus accumbens measured by in vivo microdialysis: a meta-analysis. Synapse. 2008;62:243–52.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Butelman ER, Yuferov V, Kreek MJ. κ-Opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 2012;35:587–96.
Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–35.
Ross FB, Smith MT. The intrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated. Pain. 1997;73:151–7.
Nielsen CK, Ross FB, Lotfipour S, Saini KS, Edwards SR, Smith MT. Oxycodone and morphine have distinctly different pharmacological profiles: radioligand binding and behavioural studies in two rat models of neuropathic pain. Pain. 2007;132:289–300.
Ruan X, Mancuso KF, Kaye AD. Revisiting oxycodone analgesia: a review and hypothesis. Anesthesiol Clin. 2017;35(2):e163–74. https://doi.org/10.1016/j.anclin.2017.01.022.
Vaille C, Stern G. Drug addiction: medical and social aspects in France. Bul Narc. 1954;6:2.
Bloomquist ER. The addiction potential of oxycodone (Percodan). Calif Med. 1963;99:127–30.
Maruta T, Swanson D, Finlayson R. Drug abuse and dependency in patients with chronic pain. Mayo Clin Proc. 1979;54:241–4.
•• Maruta T, Swanson DW. Problems with the use of oxycodone compound in patients with chronic pain. Pain. 1981;11:389–96 (excellent review of the problems encountered with the use of oxycodone in patients with chronic pain).
Rosenblum A, Parrino M, Schnoll SH, Fong C, Maxwell C, Cleland CM, et al. Prescription opioid abuse among enrollees into methadone maintenance treatment. Drug Alcohol Depend. 2007;90:64–71.
Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17:E119–28.
Siegal HA, Carlson RG, Kenne DR, Swora MG. Probable relationship between opioid abuse and heroin use. Am Fam Physician. 2003;67:942–5.
Grau LE, Dasgupta N, Harvey AP, Irwin K, Givens A, Kinzly ML, et al. Illicit use of opioids: is OxyContin a “gateway drug”? Am J Addict. 2007;16:166–73.
Young AM, Havens JR. Transition from first illicit drug use to first injection drug use among rural Appalachian drug users: a cross-sectional comparison and retrospective survival analysis. Addiction. 2012;107:587–96.
Pollini RA, Banta-Green CJ, Cuevas-Mota J, Metzner M, Teshale E, Garfein RS. Problematic use of prescription-type opioids prior to heroin use among young heroin injectors. Subst Abus Rehabil. 2011;2:173–80.
Centers for Disease Control and Prevention (CDC). Notes from the field: risk factors for hepatitis C virus infections among young adults—Massachusetts, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(42):1457–8.
Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. 2012;23:37–44.
Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D. “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy. 2014;25:257–66.
Carlson RG, Nahhas RW, Martins SS, Daniulaityte R. Predictors of transition to heroin use among initially non-opioid dependent illicit pharmaceutical opioid users: a natural history study. Drug Alcohol Depend. 2016;160:127–34.
Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med. 2012;367:187–9.
Muhuri PK, Gfroerer JC, Davies MC. Associations of nonmedical pain reliever use and initiation of heroin use in the United States. CBHSQ Data Review. 2013 (Aug): 1–16.
Dart RC, Surratt HL, Cicero TJ, Parrino MW, Severtson SG, Bucher-Bartelson B, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–8.
Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70(3 Suppl):S41–54.
Haertzen CA. Addiction research center inventory (ARCI): development of a general drug estimation scale. J Nerv Ment Dis. 1965;141:300–7.
Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacol. 2013;227:177–92.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91.
McAnally H. A Keynesian (demand reduction) approach to the opioid epidemic. Pain Manag. 2018. https://doi.org/10.2217/pmt-2018-0003.
Anonymous. New guidelines create an ‘Oxy-free’ ED. ED Management. 2010;22:136–7.
Uddin F. Hope in Fort Hope: first nations community is winning the battle against prescription drug abuse. Can Fam Physician. 2013;59:391–3.
American Academy of Pain Medicine. Use of opioids for the treatment of chronic pain: a statement from the American Academy of Pain Medicine. 2013. http://www.painmed.org/files/use-of-opioids-for-the-treatment-of-chronic-pain.pdf. Accessed 30 May 2018.
Interagency guideline on prescribing opioids for pain developed by the Washington state agency medical directors’ group 3rd Edition, 2015. http://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf. Accessed 30 May 2018.
Veterans Administration/Department of Defense. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. Washington: Veterans Administration; 2010. Available from: http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_fulltext.pdf. Accessed 30 May 2018.
Centers for Disease Control and Prevention. CDC guideline for prescribing opioids for chronic pain—United States. MMWR. 2016;65(1):1–49.
Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–70.
Tomasi D, Volkow ND. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb Cortex. 2014;24:935–44.
Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain. 2006;123:28–36.
Arendt-Nielsen L, Olesen AE, Staahl C, Menzaghi F, Kell S, Wong GY, et al. Analgesic efficacy of peripheral kappa-opioid receptor agonist CR665 compared to oxycodone in a multi-modal, multi-tissue experimental human pain model: selective effect on visceral pain. Anesthesiology. 2009;111:616–24.
Cepeda MS, Fife D, Ma Q, Ryan PB. Comparison of the risks of opioid abuse or dependence between tapentadol and oxycodone: results from a cohort study. J Pain. 2013;14:1227–41.
Dart RC, Cicero TJ, Surratt HL, Rosenblum A, Bartelson BB, Adams EH. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8:395–402.
Butler SF, McNaughton EC, Black RA. Tapentadol abuse potential: a postmarketing evaluation using a sample of individuals evaluated for substance abuse treatment. Pain Med. 2015;16:119–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Heath McAnally and Daniel Remillard declare no conflict of interest. Alan Kaye is on the speaker bureau for Merck and Depomed, Inc.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Other Pain
Rights and permissions
About this article
Cite this article
Remillard, D., Kaye, A.D. & McAnally, H. Oxycodone’s Unparalleled Addictive Potential: Is it Time for a Moratorium?. Curr Pain Headache Rep 23, 15 (2019). https://doi.org/10.1007/s11916-019-0751-7
Published:
DOI: https://doi.org/10.1007/s11916-019-0751-7