Abstract
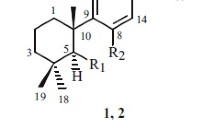
Three new megastigmanes (1–3), named annoionols A and B (1, 2) and annoionoside (3), were isolated from the leaves of Annona muricata L. (Annonaceae) together with 14 known compounds (4–17). Among the known compounds, annoionol C (4) was isolated from a natural source for the first time. The structures of all compounds were elucidated by spectroscopic and chemical analyses.




Similar content being viewed by others
References
Lannuzel A, Höglinger GU, Champy P, Michel PP, Hirsch EC, Ruberg M (2006) Is atypical parkinsonism in the Caribbean caused by the consumption of Annonaceae? J Neural Transm Suppl 70:153–157
Shitamoto J, Matsunami K, Otsuka H, Shinzato T, Takeda Y (2010) Elaeocarpionoside, a megastigmane glucoside from the leaves of Elaeocarpus japonicus Sieb. et Zucc. J Nat Med 64:104–108
Liu YP, Cai XH, Feng T, Li Y, Li XN, Luo XD (2011) Triterpene and sterol derivatives from the roots of Breynia fruticosa. J Nat Prod 74:1161–1168
Kusumi T, Ooi T, OhkuboY YabuuchiT (2006) The modified Mosher’s method and the sulfoximine method. Bull Chem Soc Jpn 79:965–980
Kasai R, Suzuno M, Asakawa J, Tanaka O (1977) Carbon-13 chemical shifts of isoprenoid-β-d-glucopyranosides and -β-d-mannopyranosides. Stereochemical influences of aglycone alcohols. Tetrahedron Lett 18:175–178
Sueyoshi E, Liu H, Matsunami K, Otsuka H, Shinzato T, Aramoto M, Takeda Y (2006) Bridelionosides A–F: megastigmane glucosides from Bridelia glauca f. balansae. Phytochemistry 67:2483–2493
Pasi S, Aligiannis N, Pratsinis H, Skaltsounis AL, Chinou LB (2009) Rhusonoside A, a new megastigmane glycoside from Rhus sylvestris, increases the function of osteoblastic MC3T3-E1 cells. Planta Med 75:163–167
Xu Y, Feng T, Cai XH, Luo XD (2009) A new C13-norisoprenoid from leaves of Alstonia scholaris. Zhongguo Tianran Yaowu 7:21–23
Zhang Z, Zhen W, Ji YP, Zhao Y, Wang CG, Hu JF (2010) Gynostemosides A–E, megastigmane glycosides from Gynostemma pentaphyllum. Phytochemistry 71:693–700
Mei W, Dai H, Wu D (2006) Isolation and identification of chemical constituents from Cephalomappa sinensis. Zhongguo Yaowu Huaxue Zazhi 16:240–243
Perez C, Trujillo JM, Almonacid LN, Navarro E, Alonso SJ (1996) New C13-norisoprenoids from Apollonias barbujana. Nat Prod Lett 8:1–6
Kuang HX, Yang BY, Xia YG, Feng WS (2008) Chemical constituents from the flowers of Datura metel L. Arch Pharm Res 31:1094–1097
Otsuka H, Yao M, Kamada K, Takeda Y (1995) Alangionosides G–M: glycosides of megastigmane derivatives from the leaves of Alangium premnifolium. Chem Pharm Bull 43:754–759
Kawakami S, Matsunami K, Otsuka H, Shinzato T, Takeda Y (2011) Crotonionosides A–G: megastigmane glycosides from leaves of Croton cascarilloides Raeuschel. Phytochemistry 72:147–153
Yu Q, Otsuka H, Hirata E, Shinzato T, Takeda Y (2002) Turpinionosides A–E: megastigmane glucosides from leaves of Turpinia ternata Nakai. Chem Pharm Bull 50:640–644
Umehara K, Hattori I, Miyase T, Ueno A, Hara S, Kageyama C (1988) Studies on the constituents of leaves of Citrus unshiu Marcov. Chem Pharm Bull 36:5004–5008
Matsunami K, Otsuka H, Takeda Y (2010) Structural revisions of blumenol C glucoside and byzantionoside B. Chem Pharm Bull 58:438–441
Mori K, Khlebnikov V (1993) Carotenoids and degraded carotenoids, VIII – Synthesis of (+)-dihydroactinidiolide, (+)- and (−)-actinidiolide, (+)- and (−)-loliolide as well as (+)- and (−)-epiloliolide. Liebigs Ann Chem 1993: 77–82
Kim, Lee SK, Kim CS, Kim KS, Moon DC (2004) Phytochemical constituents of Carpesium macrocephalum F(R). et S(AV). Arch Pharm Res 27:1029–1033
Someya Y, Kobayashi A, Kubota K (2001) Isolation and identification of trans-2- and trans-3-hydroxy-1,8-cineole glucosides from Alpinia galanga. Biosci Biotechnol Biochem 65:950–953
Mizutani K, Yuda M, Tanaka O, Saruwatari Y, Fuwa T, Jia MR, Ling YK, Pu XF (1988) Chemical studies on the Chinese traditional medicine, dangshen. I. Isolation of (Z)-3- and (E)-2-hexenyl β-d-glucosides. Chem Pharm Bull 36:2689–2690
Ly TN, Shimoyamada M, Yamauchi R (2006) Isolation and characterization of rosmarinic acid oligomers in Celastrus hindsii Benth leaves and their antioxidative activity. J Agric Food Chem 54:3786–3793
Beck MA, Haberlein H (1999) Flavonol glycosides from Eschscholtzia californica. Phytochemistry 50:329–332
Brasseur T, Angenot L (1986) Flavonol glycosides from leaves of Strychnos variabilis. Phytochemistry 25:563–564
Fukui Y, Tanaka Y, Kusumi T, Iwashita T, Nomoto K (2003) A rationale for the shift in colour towards blue in transgenic carnation flowers expressing the flavonoid 3′,5′-hydroxylase gene. Phytochemistry 63:15–23
Matsunami K, Takamori I, Shinzato T, Aramoto M, Kondo K, Otsuka H, Takeda Y (2006) Radical-scavenging activities of new megastigmane glucosides from Macaranga tanarius (L.) Müll.-Arg. Chem Pharm Bull 54:1403–1407
Yen CT, Wu CC, Lee JC, Chen SL, Morris NSL, Hsieh PW, Wu YC (2010) Cytotoxic N-(fluorenyl-9-methoxycarbonyl) (Fmoc)-dipeptides: Structure–activity relationships and synergistic studies. Eur J Med Chem 45:2494–2502
Acknowledgments
The authors are grateful for the access to the superconducting NMR instrument (JEOL ECA-600) and the Applied Biosystem QSTAR XL system ESI (NanoSpray) mass spectrometer at the Natural Science Center for Basic Research and Development (N-BARD), Hiroshima University. This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos. 22590006 and 23590130), and the Ministry of Health, Labour and Welfare. Thanks are also due to the Research Foundation for Pharmaceutical Sciences and the Takeda Science Foundation for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsushige, A., Matsunami, K., Kotake, Y. et al. Three new megastigmanes from the leaves of Annona muricata . J Nat Med 66, 284–291 (2012). https://doi.org/10.1007/s11418-011-0583-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-011-0583-1