Abstract
Purpose
The ability of two semi-mechanistic simulation approaches to predict the systemic pharmacokinetics (PK) of inhaled corticosteroids (ICSs) delivered via dry powder inhalers (DPIs) was assessed for mometasone furoate, budesonide and fluticasone propionate.
Methods
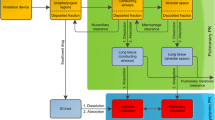
Both approaches derived the total lung doses and the central to peripheral lung deposition ratios from clinically relevant cascade impactor studies, but differed in the way the pulmonary absorption rate was derived. In approach 1, the rate of in vivo drug dissolution/absorption was predicted for the included ICSs from in vitro aerodynamic particle size distribution and in vitro drug solubility estimates measured in an in vivo predictive dissolution medium. Approach 2 derived a first order absorption rate from the mean dissolution time (MDT), determined for the test formulations in an in vitro Transwell® based dissolution system.
Results
Approach 1 suggested PK profiles which agreed well with the published pharmacokinetic profiles. Similarly, within approach 2, input parameters for the pulmonary absorption rate constant derived from dissolution rate experiments were able to reasonably predict the pharmacokinetic profiles published in literature.
Conclusion
Approach 1 utilizes more complex strategies for predicting the dissolution/absorption process without providing a significant advantage over approach 2 with regard to accuracy of in vivo predictions.







Similar content being viewed by others
Abbreviations
- A:
-
Amount of undissolved drug in GIT
- APSD:
-
Aerodynamic particle size distribution
- BUD:
-
Budesonide
- CICL:
-
Ciclesonide
- CL:
-
Clearance
- c/p ratio:
-
Central to peripheral lung deposition ratio
- Cs:
-
Saturation solubility (g/ml)
- dgeo :
-
Geometric particle size
- daero :
-
Aerodynamic particle size
- D:
-
Diffusion coefficient (cm2/s)
- DPI:
-
Dry powder inhalers
- F:
-
Fraction oral bioavailability
- FP:
-
Fluticasone propionate
- GIT:
-
Gastrointestinal tract
- GSD:
-
Geometric standard deviation
- hi :
-
Diffusion layer thickness of the particle associated with each stage i (cm)
- HFA:
-
Hydrofluoroalkane
- HPLC:
-
High performance liquid chromatography
- IVIVC:
-
In-vitro in-vivo correlation
- k:
-
Shape factor
- k12:
-
First order distribution rate constant from central to peripheral body compartment
- k21:
-
First order distribution rate constant from peripheral to central body compartment
- k10:
-
First order elimination rate constant
- ka:
-
First order absorption rate from GIT
- kaL :
-
First order absorption rate from lung
- kmuc:
-
Mucociliary clearance first order rate constant
- kpulC :
-
First order absorption rate from central lung
- kpulP :
-
First order absorption rate from peripheral lung
- LC1:
-
Drug deposited in central lung
- LC2:
-
Drug dissolved in central lung
- LP1:
-
Drug deposited in peripheral lung
- LP2:
-
Drug dissolved in peripheral lung
- Log P:
-
Logarithm of the octanol/water partition coefficient
- MAT:
-
Mean absorption time
- MDI:
-
Metered dose inhaler
- MDT:
-
Mean dissolution time
- MF:
-
Mometasone furoate
- MMAD:
-
Mass median aerodynamic diameter
- MPPD:
-
Multiple-path particle dosimetry model
- N:
-
Number of drug particles
- η :
-
Viscosity (cp)
- NGI:
-
Next generation impactor
- PIF:
-
Peak inspiratory flow
- PBPK:
-
Physiologically based pharmacokinetics
- PK:
-
Pharmacokinetic
- ρ:
-
Density of particles (g/cm3)
- r:
-
Particle radius (cm)
- rpm:
-
Rotations per minute
- Si :
-
Surface area of particle associated with each stage of the NGI, i (cm2)
- t:
-
Time (hrs)
- TLD:
-
Total lung dose
- Xd:
-
Amount dissolved (g)
- Xsum :
-
Total amount of undissolved drug (g)
- V:
-
Volume of the lung (ml)
- Vc:
-
Volume of distribution in the central compartment (L)
- VM :
-
Molecular volume (cm3/mol)
References
Hochhaus G, Möllmann H, Derendorf H, Gonzalez-Rothi RJ. Pharmacokinetic/pharmacodynamic aspects of aerosol therapy using glucocorticoids as a model. J Clin Pharmacol. 1997;37:881–92.
Hastedt JE, Bäckman P, Clark AR, Doub W, Hickey A, Hochhaus G, et al. Scope and relevance of a pulmonary biopharmaceutical classification system AAPS/FDA/USP Workshop March 16-17th, 2015 in Baltimore, MD. AAPS Open 2016 2:1. 2nd ed. Springer International Publishing; 2016;2:1.
Bäckman P, Adelmann H, Petersson G, Jones CB. Advances in Inhaled Technologies: Understanding the Therapeutic Challenge, Predicting Clinical Performance, and Designing the Optimal Inhaled Product. CPT. 2014;95:509–20.
Olsson B, Borgström L, Lundbäck H, Svensson M. Validation of a General In Vitro Approach for Prediction of Total Lung Deposition in Healthy Adults for Pharmaceutical Inhalation Products. J Aerosol Med Pulm Drug Deliv. 2013;26:355–69.
Sahib MN, Darwis Y, Khiang PK. Aerodynamic characterization of marketed inhaler dosage forms: High performance liquid chromatography assay method for the determination of budesonide. Afr J Pharm Pharmacol. 2010;4:878–84.
Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10:S28.
Ung KT, Chan H-K. Effects of ramp-up of inspired airflow on in vitro aerosol dose delivery performance for certain dry powder inhalers. Eur J Pharm Sci. Elsevier B.V; 2016;84:46–54.
Rohrschneider M, Bhagwat S, Krampe R, Michler V, Breitkreutz J, Hochhaus G. Evaluation of the Transwell System for Characterization of Dissolution Behavior of Inhalation Drugs: Effects of Membrane and Surfactant. Mol Pharm Am Chem Soc. 2015;12:2618–24.
Delvadia RR, Longest PW, Byron PR. In Vitro Tests for Aerosol Deposition. I: Scaling a Physical Model of the Upper Airways to Predict Drug Deposition Variation in Normal Humans. J Aerosol Med Pulm Drug Deliv. 2012;25:32–40.
Xi J, Longest PW. Transport and Deposition of Micro-Aerosols in Realistic and Simplified Models of the Oral Airway. Ann Biomed Eng. Kluwer Academic Publishers-Plenum Publishers. 2007;35:560–81.
Chapter <601> Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers. U.S. Pharmacopoeia-National Formulary USP NF [USP 39 NF 34]. Rockville, MD; 2016.
Delvadia RR, Wei X, Longest PW, Venitz J, Byron PR. In vitro Tests for Aerosol Deposition. IV: Simulating Variations in Human Breath Profiles for Realistic DPI Testing. - PubMed - NCBI. J Aerosol Med Pulm Drug Deliv. 2016;29:196–206.
Yeh HC, Schum GM. Models of human lung airways and their application to inhaled particle deposition. Bull Math Biol. 1980;42:461–80.
May S, Jensen B, Weiler C, Wolkenhauer M, Schneider M, Lehr C-M. Dissolution testing of powders for inhalation: influence of particle deposition and modeling of dissolution profiles. Pharm Res. 2014;31:3211–24.
Kosoglou T, Hubbell J, Kantesaria B, Hanson ME, Cutler DL. An evaluation of the systemic bioavailability of mometasone furoate (MF) after oral inhalation from a MF/formoterol fumarate metered-dose inhaler versus an MF dry-powder inhaler in healthy subjects. CPDD. 2014;3:229–34.
Loo JCK, Riegelman S. New Method for Calculating the Intrinsic Absorption Rate of Drugs. J Pharm Sci. 1968;57:918–28.
Affrime MB, Cuss F, Padhi D, Wirth M, Pai S, Clement RP, et al. Bioavailability and metabolism of mometasone furoate following administration by metered-dose and dry-powder inhalers in healthy human volunteers. J Clin Pharmacol. 2000;40:1227–36.
Weber B, Hochhaus G. A Pharmacokinetic Simulation Tool for Inhaled Corticosteroids. AAPS J. 2013;15:159–71.
Costa P, Manuel J, Lobo S. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33.
Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL; http://www.R-project.org/.
Moellmann H, Krishnaswami M, Tang HD, Falcoz C, Daley-Yates P, Krieg M, et al. Single dose and steady state pharmcokinetic and pharmacodynamic evaluation of therapeutically clinically equivalent doses of inhaled fluticasone propionate and budesonide, given as diskus or turbuhaler dry powder inhalers to healthy subjects. J Clin Pharmacol. 2001;41:1329–38.
Lähelmä S, Kirjavainen M, Kela M, Herttuainen J, Vahteristo M, Silvasti M, et al. Equivalent lung deposition of budesonide in vivo: a comparison of dry powder inhalers using a pharmacokinetic method. Br J Clin Pharmacol. 2005;59:167–73.
Maffia A. Sandoz Citizen Petition. Docket ID FDA--P. 2016 :1–20.
Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80:372–80.
Bhatt H, Naik B, Dharamsi A. Solubility Enhancement of Budesonide and Statistical Optimization of Coating Variables for Targeted Drug Delivery. J Pharm. Hindawi Publishing Corporation. 2014:1–13.
Thorsson L, Edsbäcker S, Källén A, Löfdahl CG. Pharmacokinetics and systemic activity of fluticasone via Diskus and pMDI, and of budesonide via Turbuhaler. Br J Clin Pharmacol. 2001;52:529–38.
Thorsson L, Edsbäcker S, Conradson TB. Lung deposition of budesonide from Turbuhaler is twice that from a pressurized metered-dose inhaler P-MDI. Eur Respir J. 1994;7:1839–44.
Allen A, Bareille PJ, Rousell VM. Fluticasone Furoate, a Novel Inhaled Corticosteroid, Demonstrates Prolonged Lung Absorption Kinetics in Man Compared with Inhaled Fluticasone Propionate. Clin Pharmacokinet. Springer International Publishing AG. 2012;52:37–42.
Brindley C, Falcoz C, Mackie A, Bye A. Absorption Kinetics after Inhalation of Fluticasone Propionate via the Diskhaler®, Diskus® and Metered-Dose Inhaler in Healthy Volunteers. Clin Pharmacokinet. 2000;59(Suppl.1):1–8.
Derendorf H, Hochhaus G, Krishnaswami S. Systemic disposition and effects of inhaled corticosteroids. Inhaled Steroids in Asthma, Lung Biology in Health and Disease, Volume 163. In: Schleimer RP, O'Byrne PM, Szefler SJ, Brattsand R, editors. Marcel Dekker, New York; 2001. p. 247–70.
Singh SD, Whale C, Houghton N, Daley-Yates P, Kirby SM, Woodcock AA. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in chronic obstructive pulmonary disease. Br J Clin Pharmacol. Wiley-Blackwell. 2003;55:375–81.
Bäckman P, Tehler U, Olsson B. Predicting Exposure After Oral Inhalation of the Selective Glucocorticoid Receptor Modulator, AZD5423, Based on Dose, Deposition Pattern, and Mechanistic Modeling of Pulmonary Disposition. J Aerosol Med Pulm Drug Deliv. 2017;30:108–17.
Dokoumetzidis A, Macheras P. A century of dissolution research: From Noyes and Whitney to the Biopharmaceutics Classification System. Int J Pharm. 2006;321:1–11.
Hixson AW, Crowell JH. Dependence of reaction velocity upon surface and agitation I-Theoretical considerations. Ind Eng Chem. 1931;23:923–31.
Higuchi WI, Rowe EL, Hiestand EN. Dissolution rates of finely divided drug powders II. Micronized methylprednisolone. J Pharm Sci. Wiley Subscription Services, Inc., A Wiley Company. 1963;52:162–4.
Boger E, Evans N, Chappell M, Lundqvist A, Ewing P, Wigenborg A, et al. Systems Pharmacology Approach for Prediction of Pulmonary and Systemic Pharmacokinetics and Receptor Occupancy of Inhaled Drugs. CPT Pharmacometrics Syst Pharmacol. 2016;5:201–10.
Molina DK, DiMaio VJM. Normal Organ Weights in Men Part II-The Brain, Lungs, Liver, Spleen, and Kidneys. Am J Forensic Med Pathol. 2012;33:368–72.
Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, et al. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol. 1986;60:532–8.
Whelan GJ, Blumer JL, Martin RJ, Szefler SJ, Asthma Clinical Research Network and the Pediatric Pharmacology Research Unit Network. Fluticasone propionate plasma concentration and systemic effect: effect of delivery device and duration of administration. J Allergy Clin Immunol. 2005;116:525–30.
Hayduk W, Laudie H. Prediction of diffusion coefficients for nonelectrolytes in dilute aqueous solutions. AICHE J. 1974;20:611–5.
Zhao YH, Abraham MH, Zissimos AM. Fast calculation of van der Waals volume as a sum of atomic and bond contributions and its application to drug compounds. J Org Chem. 2003;68:7368–73.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Fitting MF’s absorption profile to the Nernst-Brunner Equation
The absorption profile of MF derived by deconvouting the PK profile (see main text) was fitted to the Nernst-Brunner equation. Using information from cascade impactor studies (amount of drug deposited on stages 1 through 7) saturation solubility Cs could be easily determined as only unknown parameter.
Xsum - total amount of undissolved drug (g).
D – diffusion coefficient (cm2/s).
Si – surface area of particle associated with each stage of the NGI, i(cm2), calculated from amounts deposited on a given stage by applying eq. 3–6.
hi – diffusion layer thickness of the particle associated with each stage i (cm).
Cs – saturation solubility (g/ml).
Xd – amount dissolved (g).
V – volume (840 ml, representing the lung volume (37)).
r: radius.
The surface area (Si) associated with particles on a given NGI stage was calculated from the amounts of undissolved drug present on a given NGI stage (Xi), the starting geometric diameter (dgeo) of particles of a given NGI stage, the related starting radius of particles (r), the remaining radius during dissolution of the particles (ri), the number of particles (Ni), the density of particles (ρ) as follows:
Considering that ICSs are non-ionized, the diffusion coefficient D in eq. 2 was based on the Hayduk-Laudie equation (equation 7) (40) assuming that the viscosity η of water is similar to that of the lung, as data for the lung environment are not available.
The molecular volume VM for the corticosteroids was calculated using the approach described by Zhao et al. (41). Fitting was performed using the differential form of the Nernst-Brunner equation (eq. 2) with a dt of 0.01 min. The radii of the particles (ri) and their surface areas (Si) were updated for each time point by calculating the change in amount dissolved and consequently the reduction in radius and surface area of particles at each time t. The shape of the particles was assumed to be spherical.
Appendix 2: Differential Equations describing Approach 1
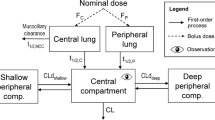
Change in undissolved drug (LC1) in central lung:
Parameters related to the Nernst-Brunner equation are identical to those of eq. 2, subscript C indicates central lung compartment; kmuc represents mucociliary clearance rate; LC1 amount of solid drug remaining in the central compartment. Si,c – surface area of particle associated with each stage i of the NGI (cm2). Surface area was not treated as constant but adjusted during the dissolution process as outlined in eqs. 2–7.
hi,c – diffusion layer thickness of the particle associated with each stage i (cm); Cs – saturation solubility (g/ml); D – diffusion coefficient (cm2/s); XdC: amount dissolved in central lung; V: Volume of lung (840 ml equivalent to the aqueous volume of the lung (37)).
Change in dissolved drug (LC2) in central lung:
Parameters related to the Nernst-Brunner equation are identical to those of eq. 2; subscript C indicates central lung compartment, kpulC: absorption rate from central lung, set to 10 h−1 to achieve sink conditions. LC2 amount of dissolved drug remaining in the central compartment. V: Volume of lung (840 ml equivalent to the aqueous volume of the lung (37)).
Change in undissolved drug (LP1) in peripheral lung:
Parameters related to the Nernst-Brunner equation are identical to those of eq. 2, subscript P indicates peripheral lung compartment; LP1 amount of solid drug remaining in the peripheral compartment; Si,p – surface area of particles associated with each stage of the NGI, i(cm2). Surface area was not treated as constant but adjusted during the dissolution process as outlined in equations 2–7); hi,p – diffusion layer thickness of the particle associated with each stage i (cm); Cs – saturation solubility (g/ml); D – diffusion coefficient (cm2/s); Xdp: amount dissolved in peripheral lung; Volume of lung (840 ml equivalent to the aqueous volume of the lung (37)).
Change in dissolved drug (LP2) in peripheral lung:
Parameters related to the Nernst-Brunner equation are identical to those of eq. 2; subscript P indicates peripheral lung compartment, kpulP indicates the absorption rate from peripheral lung, which was set to 20 h−1 to achieve sink conditions. LP2 represents the amount of dissolved drug remaining in the peripheral compartment, while V denotes the volume of the lung (840 ml).
Change in undissolved drug (A) present in GIT (only relevant for BUD in the absence of charcoal):
ka-first order oral absorption rate constant, A-amount of undissolved drug in GIT, kmuc-mucociliary clearance rate, LC1- amount of undissolved drug remaining in the central lung.
Change in drug (X) present in the central systemic compartment:
k12, k21-intercompartmental rate constants, describing two compartment body model, F-oral bioavailability, P-amount in peripheral systemic compartment, A-amount of drug in GIT tract.
Change in drug (P) present in the peripheral systemic compartment:
k12, k21-intercompartmental rate constants, describing two compartment body model
Appendix 3: Differential Equations describing Approach 2
The following differential equations describe modeling approach 2 (see also Scheme 1 and 2):
Change in undissolved drug (LC1) in the central lung compartment:
kaL: absorption rate from lung. LC1-amount of undissolved drug remaining in the central compartment; Kmuc-mucociliary clearance rate.
Change in undissolved drug in the peripheral lung compartment:
kaL: absorption rate from lung. LC1-amount of undissolved drug remaining in the peripheral compartment.
Change in drug (X) present in the central systemic compartment:
k12, k21-intercompartmental rate constants, describing two compartment body model, F-oral bioavailability, P-amount in peripheral systemic compartment, A-amount of drug in GIT tract; kaL: absorption rate from lung; LC1-amount of undissolved drug remaining in the central lung; LP1-amount of undissolved drug remaining in the peripheral lung; ka-absorption rate constant from GIT.
Change in drug (P) present in the peripheral systemic compartment:
Rights and permissions
About this article
Cite this article
Bhagwat, S., Schilling, U., Chen, MJ. et al. Predicting Pulmonary Pharmacokinetics from In Vitro Properties of Dry Powder Inhalers. Pharm Res 34, 2541–2556 (2017). https://doi.org/10.1007/s11095-017-2235-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2235-y