Abstract
The biogeochemical cycles of H, C, N, O and S are coupled via biologically catalyzed electron transfer (redox) reactions far from thermodynamic equilibrium. In this paper I examine the evolution of the structural motifs responsible for redox reactions (the biological “transistors”) across the tree of life, and the photogeochemical reactions on minerals that ultimately came to be the driving force for these biological reactions.
Similar content being viewed by others
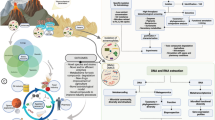
All life on Earth uses six elements to form biological macromolecules: H, C, N, O, P and S (Schlesinger and Berhardt 2013). The macromolecules themselves are derived from the extraction of energy from the environment that is used to generate a series of coupled oxidation/reduction reactions, in which electrons are transferred, often with protons, to form the core of metabolism: proton coupled electron transfer reactions (Falkowski et al. 2008). Although the classical Boltzmann concept of entropy does not strictly apply in an open system, life and its attendant proton coupled electron transfer reactions are far from thermodynamic equilibrium. The result is that life is literally “electric”; it moves electrons associated with specific gases across the globe. The connections between metabolic processes that allow the continuous recycling of the electrons are made possible by two planetary “wires”: the oceans and the atmosphere. The primary source of electrons at present is liquid water, however in the late Hadean or early Archean, it may have been H2 or H2S. The primary driving force for extracting electrons in the biosphere is light (Falkowski 2007) (Fig. 1).
Over the past few years, together with my colleagues at Rutgers, we have taken a two track approach to understanding the evolution of biological redox reactions far from thermodynamic equilibrium. In a “top down” approach, we have searched for patterns in either primary amino acid sequence or topology in proteins that catalyze electron transfer reactions (the oxidoreductases; Enzyme Commission number 1) (Harel et al. 2012). Topological analyses focused on proteins that contain transition metals in the active sites; there are fewer than 500 such proteins, not including orthologues or paralogues. Inspection of all such proteins across the tree of life revealed that the most abundant transition metal, by far, is iron, either as a free metal ion, as a sulfide, or a haeme; these three basic structures account for ~ 70 % of all metal containing oxidoreductases (Harel et al. 2014). The second most commonly used metal is copper (14 %), followed by molybdenum (6 %). The protein/metal complexes function as biological transistors, transferring electrons in a binary fashion resulting from conformational changes in the active site.
Topological analysis was developed by comparing the structural features within a radius of 15 Å from the metal center (Kim et al. 2013a, b). Statistical analysis revealed this radius was sufficiently large to capture differences in topological features, but small enough to preclude interference of structures far away from the active site. For the sake of simplicity, we considered only secondary structures, which can be assigned to a spatial Euclidian vector space in the context of a loop, a helix, or a sheet (Senn et al. 2014). The results of this analysis allowed for the construction of a phylogenetic tree of proteins with the quasi-Bayesian assumption that the most rapidly evolving structures are loops, or inherently disordered peptides. The base of the phylogenetic tree contains a ferredoxin type of domain, with a C X X C XX C…. C fold, where X is any amino acid residue, and C is cysteine. The folds of all ferredoxins are chiral and right handed, and the cysteines are coordinated to iron atoms in a Fe4S4 cluster (Kim et al. 2012). There is possibly an earlier, simpler, and potentially related fold in desulforedoxin, where a metal (usually iron) is bound to two cysteine residues. Regardless, these relatively simple folds gave rise to enzymes capable of using H2 as a source of reductant. Deriving from this topological feature, gene duplication and mutation followed by selection gave rise to a series of more structurally complex domains that rapidly diverged into sheet and helix supergroups. Three critical groups of proteins arose in the helix supergroup: nitrogenases, photosynthetic reaction centers and respiratory complexes. The last protein family to have evolved appears to have been the cytochrome c oxidases, which became critical for aerobic respiration. The sheet superfamily contains several enzymes that detoxify reactive oxygen species, which presumably formed in the Archean era from direct, high energy photoreactions with water and ice, prior to the origin of oxygenic photosynthesis .
The distribution of the secondary structural folds does not correlate with the distribution of transition metals. Furthermore, network analyses of both structural features and primary sequences strongly suggests that the evolution of the oxidoreductases was polyphyetic (Harel et al. 2014). Indeed, at least ten proteins appear to have been recruited independently to become oxidoreductases. The earliest origins of the photobiochemical reactions remain elusive, however structural analyses suggest that the earliest substrate was probably H2.
The structural analyses of oxidoreductases provide clues as to how the earliest life forms evolved simple bioelectronic devices. However, that approach does not explain how the devices operated far from thermodynamic equilibrium. The successful operation of the devices in living cells requires a power supply. In this context, a power supply is an energy source and a transduction system that converts the energy source to a source of power that can generate an electrical potential.
To further examine how photobiochemical reactions potentially evolved to provide a power supply, we took a second, “bottom-up” approach and examined the potential photochemical reactivity of certain likely common minerals with light. One mineral of interest is siderite, FeCO3. Siderite was probably an abundant sedimentary mineral in shallow waters of the early Archean, when soluble iron was available, oxygen concentrations were extremely low, and carbon dioxide concentrations were presumably very high. Density function calculations suggest the molecule has an antibonding orbital at ~ 4.8 eV, corresponding to light at 270 nm. We synthesized siderite under strictly anoxic conditions, and exposed the mineral in aqueous suspensions of the mineral to UV radiation at specific band gaps. This led to the photochemical oxidation of siderite, forming first a non-magnetic mineral and finally magnetite. The sink for the electrons appears to have been protons and H2 was also evolved (Kim et al. 2013a, b). The reaction requires two photons to generate two electrons independently; consequently the quantum yield is low. However, it works. The effective cross sections for the photochemical oxidation of siderite in the ultra violet potentially supplied electrons for life in the Archean oceans at fluxes comparable if not exceeding that from hydrothermal vents. This and other photogeochemical reactions involving minerals almost certainly played a significant role in fueling the earliest life forms on Earth.
We remain a long way from understanding the contingencies and conditions that led to the “spark” of life, however we have also come a long way over the past two decades. Protein structures are increasingly allowing us to glimpse into the deep past to examine the motifs that have been utilized repeatedly over time. Like basic structural elements in architecture, simple folds in the oxidoreductases have been utilized and modified, elaborated and retained. Similarly, regardless of whether life on this planet began in a “warm little pond” (Darwin 1871), a hydrothermal vent (Martin et al. 2008), or in some other environment with a chemical gradient (Lane et al. 2010), ultimately it utilized light from the Sun to provide the overwhelming source of energy for the biosphere. How light energy was converted to chemical energy is not known. However, it is known that solar excitation of some minerals can drive electron transfer reactions far from thermodynamic equilibrium. This abiotic, photogeochemical production of high-energy products potentially provided prebiotic substrates for the subsequent evolution of biological catalysis. Closing the gaps between the evolution of life’s power supply and the bioelectronic devices that access the electrons to form a global circuit is the focus of our research.
References
Darwin C (1871) The descent of man, and selection in relation to sex. J. Murray, London
Falkowski PG, Fenchel T, Delong EF (2008) The microbial engines that drive Earth’s biogeochemical cycles. Science 320(5879):1034–1039
Falkowski PG, Raven JA (2007) Aquatic Photosynthesis, 2nd edn. Princeton University Press, Princeton
Harel A, Bromberg Y, Falkowski P, Bhattacharya D (2014) Evolutionary history of redox metal-binding domains across the tree of life. Proc Natl Acad Sci U S A 111:7042–7047
Harel A, Falkowski P, Bromberg Y (2012) TrAnsFuSE refines the search for protein function: oxidoreductases. Integr Biol: Quant Biosci Nano Macro 4(7):765–777
Kim J, Senn S, Harel A, Jelen B, Falkowski P (2013) Discovering the electronic circuit diagram of life: structural relationships among transition metal binding sites in oxidoreductases. Philos Trans R Soc B 368: doi: 10.1098/rstb.2012.0257
Kim JD, Rodriguez-Granillo A, Case DA, Nanda V, Falkowski PG (2012) Energetic selection of topology in ferredoxins. PLoS Comput Biol 8(4):e1002463
Kim JD, Yee N, Nanda V, Falkowski PG (2013b) Anoxic photochemical oxidation of siderite generates molecular hydrogen and iron oxides. Proc Natl Acad Sci U S A 110(25):10073–10077
Lane N, Allen JF, Martin W (2010) How did LUCA make a living? Chemiosmosis in the origin of life. Bioessays 32(4):271–280
Martin W, Baross J, Kelley D, Russell MJ (2008) Hydrothermal vents and the origin of life. Nat Rev Microbiol 6(11):805–814
Schlesinger W, Berhardt E (2013) Biogeochemistry: An Analysis of Global Change. Elsevier, Amsterdam
Senn S, Nanda V, Falkowski P, Bromberg Y (2014) Function-based assessment of structural similarity measurements using metal co-factor orientation. Proteins 82(4):648–656
Acknowledgments
Our research is supported by the Gordon and Betty Moore Foundation Grant GBMF2807 to Paul Falkowski and by Grant 0940187 from the National Science Foundation Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falkowski, P.G. From Light to Life. Orig Life Evol Biosph 45, 347–350 (2015). https://doi.org/10.1007/s11084-015-9441-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-015-9441-6