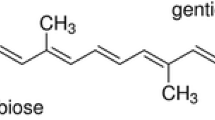
Abstract
Glaucoma is a group of neurodegenerative diseases characterized by the progressive loss of retinal ganglion cells (RGCs) and optic nerve fibers. Microglial activation has been shown to be deleterious to RGCs and may participate in the progression of glaucoma. Crocin, one of the major active ingredients in saffron, has been found to inhibit microglial activation. However, the mechanism remains unclear. The aim of this study was to investigate whether crocin can inhibit lipopolysaccharide (LPS)-induced microglial activation and to clarify the mechanisms involved. The influence of crocin on primary RGCs and LPS-stimulated BV2 microglial cells survival was determined by the MTT and lactate dehydrogenase assays, or by flow cytometry. BV2 cells were pretreated with various concentrations of crocin for 2 h followed by 1 μg/mL LPS stimulation. Microglial markers and pro-inflammatory mediators were assessed by real-time PCR, western blot and ELISA. Furthermore, CX3CR1 expression was detected and the underlying mechanism was examined. The concentrations of crocin ranged from 0.1 to 1 μM, and did not show any cytotoxicity in RGC and BV2 cells. After crocin pretreatment, the expression of microglial markers (CD11b and Iba-1) and pro-inflammatory mediators (iNOS, COX-2, IL-1β, and TNF-α) induced by LPS were significantly decreased in a dose-dependent manner. Additionally, CX3CR1 expression was remarkably increased by crocin via the suppression of NF-κB/Yin Yang 1 (YY1) signaling in BV2 cells. In conclusion, crocin effectively suppresses microglial activation and upregulates CX3CR1 expression by suppressing NF-κB/YY1 signaling.





Similar content being viewed by others
References
Trost A, Motloch K, Bruckner D, Schroedl F, Bogner B, Kaser-Eichberger A, Runge C, Strohmaier C, Klein B, Aigner L, Reitsamer HA (2015) Time-dependent retinal ganglion cell loss, microglial activation and blood–retina-barrier tightness in an acute model of ocular hypertension. Exp Eye Res 136:59–71
Wang L, Cioffi GA, Cull G, Dong J, Fortune B (2002) Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci 43:1088–1094
Bosco A, Steele MR, Vetter ML (2011) Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol 519:599–620
Walton NM, Sutter BM, Laywell ED, Levkoff LH, Kearns SM, Marshall GP 2nd, Scheffler B, Steindler DA (2006) Microglia instruct subventricular zone neurogenesis. Glia 54:815–825
Fourgeaud L, Boulanger LM (2007) Synapse remodeling, compliments of the complement system. Cell 131:1034–1036
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, Sher A, Litke AM, Lambris JD, Smith SJ, John SW, Barres BA (2007) The classical complement cascade mediates CNS synapse elimination. Cell 131:1164–1178
Santiago AR, Baptista FI, Santos PF, Cristovao G, Ambrosio AF, Cunha RA, Gomes CA (2014) Role of microglia adenosine A(2A) receptors in retinal and brain neurodegenerative diseases. Mediators Inflamm 2014:465694
Bosco A, Crish SD, Steele MR, Romero CO, Inman DM, Horner PJ, Calkins DJ, Vetter ML (2012) Early reduction of microglia activation by irradiation in a model of chronic glaucoma. PLoS One 7:e43602
Bosco A, Romero CO, Breen KT, Chagovetz AA, Steele MR, Ambati BK, Vetter ML (2015) Neurodegeneration severity can be predicted from early microglia alterations monitored in vivo in a mouse model of chronic glaucoma. Dis Model Mech 8:443–455
Wang K, Peng B, Lin B (2014) Fractalkine receptor regulates microglial neurotoxicity in an experimental mouse glaucoma model. Glia 62:1943–1954
Cardona SM, Mendiola AS, Yang YC, Adkins SL, Torres V, Cardona AE (2015) Disruption of fractalkine signaling leads to microglial activation and neuronal damage in the diabetic retina. ASN Neuro 7:1–18
Khazdair MR, Boskabady MH, Hosseini M, Rezaee R, Tsatsakis AM (2015) The effects of Crocus sativus (saffron) and its constituents on nervous system: a review. Avicenna J Phytomed 5:376–391
Bukhari SI, Pattnaik B, Rayees S, Kaul S, Dhar MK (2015) Safranal of Crocus sativus L. inhibits inducible nitric oxide synthase and attenuates asthma in a mouse model of asthma. Phytother Res 29:617–627
Linardaki ZI, Orkoula MG, Kokkosis AG, Lamari FN, Margarity M (2013) Investigation of the neuroprotective action of saffron (Crocus sativus L.) in aluminum-exposed adult mice through behavioral and neurobiochemical assessment. Food Chem Toxicol 52:163–170
Bolhassani A, Khavari A, Bathaie SZ (2014) Saffron and natural carotenoids: biochemical activities and anti-tumor effects. Biochim Biophys Acta 1845:20–30
Abdullaev FI, Riveron-Negrete L, Caballero-Ortega H, Manuel Hernandez J, Perez-Lopez I, Pereda-Miranda R, Espinosa-Aguirre JJ (2003) Use of in vitro assays to assess the potential antigenotoxic and cytotoxic effects of saffron (Crocus sativus L.). Toxicol In Vitro 17:731–736
Qi Y, Chen L, Zhang L, Liu WB, Chen XY, Yang XG (2013) Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp Eye Res 107:44–51
Chen L, Qi Y, Yang X (2015) Neuroprotective effects of crocin against oxidative stress induced by ischemia/reperfusion injury in rat retina. Ophthalmic Res 54:157–168
Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, Jung WS, Cho KH, Park JH, Kang I, Hong JW, Lee EH (2010) Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol 648:110–116
Wang K, Zhang L, Rao W, Su N, Hui H, Wang L, Peng C, Tu Y, Zhang S, Fei Z (2015) Neuroprotective effects of crocin against traumatic brain injury in mice: involvement of notch signaling pathway. Neurosci Lett 591:53–58
Winzeler A, Wang JT (2013) Purification and culture of retinal ganglion cells from rodents. Cold Spring Harb Protoc 2013:643–652
Duan M, Yao H, Cai Y, Liao K, Seth P, Buch S (2014) HIV-1 Tat disrupts CX3CL1–CX3CR1 axis in microglia via the NF-kappaBYY1 pathway. Curr HIV Res 12:189–200
Yang F, Wu L, Guo X, Wang D, Li Y (2013) Improved retinal ganglion cell survival through retinal microglia suppression by a chinese herb extract, triptolide, in the DBA/2 J mouse model of glaucoma. Ocul Immunol Inflamm 21:378–389
Robson MJ, Turner RC, Naser ZJ, McCurdy CR, Huber JD, Matsumoto RR (2013) SN79, a sigma receptor ligand, blocks methamphetamine-induced microglial activation and cytokine upregulation. Exp Neurol 247:134–142
da Kim J, Kim YS (2015) Trimethyltin-induced microglial activation via NADPH oxidase and MAPKs pathway in BV-2 microglial cells. Mediators Inflamm 2015:729509
Qiu LL, Ji MH, Zhang H, Yang JJ, Sun XR, Tang H, Wang J, Liu WX (2016) NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun 51:109–118
Brown GC, Neher JJ (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol 41:242–247
Kim M, Kim SO, Lee M, Lee JH, Jung WS, Moon SK, Kim YS, Cho KH, Ko CN, Lee EH (2014) Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid beta and interferon-gamma in rat brain microglia. Eur J Pharmacol 740:504–511
Stence N, Waite M, Dailey ME (2001) Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33:256–266
Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP (2010) Protracted downregulation of CX3CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun 24:1190–1201
Boddeke EW, Meigel I, Frentzel S, Biber K, Renn LQ, Gebicke-Harter P (1999) Functional expression of the fractalkine (CX3C) receptor and its regulation by lipopolysaccharide in rat microglia. Eur J Pharmacol 374:309–313
Amin A, Hamza AA, Daoud S, Khazanehdari K, Hrout AA, Baig B, Chaiboonchoe A, Adrian TE, Zaki N, Salehi-Ashtiani K (2016) Saffron-based crocin prevents early lesions of liver cancer: in vivo, in vitro and network analyses. Recent Pat Anti-Cancer Drug Discovery 11:121–133
Ishizuka F, Shimazawa M, Umigai N, Ogishima H, Nakamura S, Tsuruma K, Hara H (2013) Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol 703:1–10
Ding Q, Zhong H, Qi Y, Cheng Y, Li W, Yan S, Wang X (2013) Anti-arthritic effects of crocin in interleukin-1beta-treated articular chondrocytes and cartilage in a rabbit osteoarthritic model. Inflamm Res 62:17–25
Xiao LJ, Chen YY, Lin P, Zou HF, Lin F, Zhao LN, Li D, Guo L, Tang JB, Zheng XL, Yu XG (2012) Hypoxia increases CX3CR1 expression via HIF-1 and NFkappaB in androgen-independent prostate cancer cells. Int J Oncol 41:1827–1836
Gan AM, Butoi ED, Manea A, Simion V, Stan D, Parvulescu MM, Calin M, Manduteanu I, Simionescu M (2013) Inflammatory effects of resistin on human smooth muscle cells: up-regulation of fractalkine and its receptor, CX3CR1 expression by TLR4 and Gi-protein pathways. Cell Tissue Res 351:161–174
Meucci O, Fatatis A, Simen AA, Miller RJ (2000) Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA 97:8075–8080
He Y, Casaccia-Bonnefil P (2008) The Yin and Yang of YY1 in the nervous system. J Neurochem 106:1493–1502
Deng Z, Cao P, Wan MM, Sui G (2010) Yin Yang 1: a multifaceted protein beyond a transcription factor. Transcription 1:81–84
Karki P, Webb A, Smith K, Johnson J Jr, Lee K, Son DS, Aschner M, Lee E (2014) Yin Yang 1 is a repressor of glutamate transporter EAAT2, and it mediates manganese-induced decrease of EAAT2 expression in astrocytes. Mol Cell Biol 34:1280–1289
Bernard M, Voisin P (2008) Photoreceptor-specific expression, light-dependent localization, and transcriptional targets of the zinc-finger protein Yin Yang 1 in the chicken retina. J Neurochem 105:595–604
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81273902).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, B., Huo, F., Zhu, Z. et al. Crocin Upregulates CX3CR1 Expression by Suppressing NF-κB/YY1 Signaling and Inhibiting Lipopolysaccharide-Induced Microglial Activation. Neurochem Res 41, 1949–1957 (2016). https://doi.org/10.1007/s11064-016-1905-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1905-1