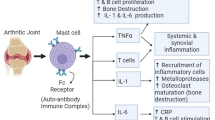
Abstract
Rheumatoid arthritis (RA) is an auto-immune inflammatory disorder of the synovial lining of joints marked by immune cells infiltration and hyperplasia of synovial fibroblasts which results in articular cartilage destruction and bone erosion. The current review will provide comprehensive information and results obtained from the recent research on the phytochemicals which were found to have potential anti-arthritic activity along with the molecular pathway that were targeted to control RA progression. In this review, we have summarized the scientific data from various animal studies about molecular mechanisms, possible side effects, associations with conventional therapies, and the role of complementary and alternative medicines (CAM) for RA such as ayurvedic medicines in arthritis. In the case of RA, phytochemicals have been shown to act through different pathways such as regulation of inflammatory signaling pathways, T cell differentiation, inhibition of angiogenic factors, induction of the apoptosis of fibroblast-like synoviocytes (FLS), inhibition of autophagic pathway by inhibiting High-mobility group box 1 protein (HMGB-1), Akt/ mTOR pathway and HIF-1α mediated Vascular endothelial growth (VEGF) expression. Also, osteoclasts differentiation is inhibited by down-regulating the VEGF expression by decreasing the accumulation of the ARNT (Aryl Hydrocarbon Receptor Nuclear Translocator)-HIF-1α complex Although phytochemicals have shown to exert potential anti-arthritic activity in many animal models and further clinical data is needed to confirm their safety, efficacy, and interactions in humans.





Similar content being viewed by others
Abbreviations
- bFGF:
-
Basic fibroblast growth factor
- CAM:
-
Complementary and alternative medicine
- CXCR4:
-
CXC chemokine receptor 4
- DNMT:
-
DNA methyltransferase
- ERK:
-
Extracellular-signal-regulated kinase
- FLS:
-
Fibroblast-like synoviocytes
- FOXP3:
-
Forkhead box P3
- GSK3B:
-
Glycogen synthase kinase 3B
- HMGB-1:
-
High-mobility group box 1 protein
- MALAT1:
-
Metastasis associated lung adenocarcinoma transcript 1
- MAPK:
-
Mitogen activated protein kinase
- MHC:
-
Major histocompatibility complex
- OPG:
-
Osteoprotegerin
- RAGE:
-
Receptor for advanced glycation end products
- RANKL:
-
Receptor activator of nuclear factor-κB ligand
- ROR:
-
Retinoic orphan receptor
- STAT3:
-
Signal transducer and activator of transcription 3
- UAA:
-
Ursolic acid-3-acetate
- VEGF:
-
Vascular endothelial growth factor
References
Nathan C (2002) Points of control in inflammation. Nature 420:846–852
Mohammed FF, Smookler DS, Khokha R (2003) Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis 62:ii43–ii47
Medzhitov R (2008) Origin and physiological roles of inflammation. Nature 454:428–435. https://doi.org/10.1038/nature07201
Lim H, Kim HP (2007) Inhibition of mammalian collagenase, matrix metalloproteinase-1, by naturally-occurring flavonoids. Planta Med 73:1267–1274
Dudics S, Langan D, Meka RR et al (2018) Natural products for the treatment of autoimmune arthritis: their mechanisms of action, targeted delivery, and interplay with the host microbiome. Int J Mol Sci. https://doi.org/10.3390/ijms19092508
Firestein GS (2003) Evolving concepts of rheumatoid arthritis. Nature 423:356–361
Silman AJ, Pearson JE (2002) Epidemiology and genetics of rheumatoid arthritis. Arthritis Res 4:S265–S272. https://doi.org/10.1186/ar578
Helmick CG, Felson DT, Lawrence RC et al (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I Arthritis Rheum 58:15–25. https://doi.org/10.1002/art.23177
Chopra A (2000) Ayurvedic medicine and arthritis. Rheum Dis Clin N Am 26:133–144
Press D (2015) Toward the integration and advancement of herbal medicine : a focus on traditional Indian medicine. Bot: Targets Therapy 5:33–44
Chopra A, Doiphode VV (2002) Ayurvedic medicine: core concept, therapeutic principles, and current relevance. Med Clin 86:75–89
McInnes IB, Schett G (2007) Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol 7:429–442. https://doi.org/10.1038/nri2094
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219
Rantapää-Dahlqvist S, De Jong BAW, Berglin E et al (2003) Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 48:2741–2749
Schröder AE, Greiner A, Seyfert C, Berek C (1996) Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci 93:221–225
Nadkarni S, Mauri C, Ehrenstein MR (2007) Anti-TNF-α therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-β. J Exp Med 204:33–39. https://doi.org/10.1084/jem.20061531
Tolboom TCA, Van Der Helm-Van Mil AHM, Nelissen RGHH et al (2005) Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum 52:1999–2002. https://doi.org/10.1002/art.21118
Lee S-Y, Kwok S-K, Son H-J et al (2013) IL-17-mediated Bcl-2 expression regulates survival of fibroblast-like synoviocytes in rheumatoid arthritis through STAT3 activation. Arthritis Res Ther 15:1–10
Ziolkowska M, Kurowska M, Radzikowska A et al (2002) High levels of osteoprotegerin and soluble receptor activator of nuclear factor κB ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor α treatment. Arthritis Rheum 46:1744–1753. https://doi.org/10.1002/art.10388
Shigeyama Y, Pap T, Kunzler P et al (2000) Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum Off J Am Coll Rheumatol 43:2523–2530
Edwards SW, Hallett MB (1997) Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today 18:320–324
Veale DJ, Fearon U (2006) Inhibition of angiogenic pathways in rheumatoid arthritis: potential for therapeutic targeting. Best Pract Res Clin Rheumatol 20:941–947
Guo H, Callaway JB, Ting JPY (2015) Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 21:677–687
Sharma D, Kanneganti T-D (2016) The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol 213:617–629
Mangan MSJ, Olhava EJ, Roush WR et al (2018) Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17:588–606
Shin MS, Kang Y, Wahl ER et al (2019) Macrophage migration inhibitory factor regulates U1 small nuclear RNP immune complex–mediated activation of the NLRP3 inflammasome. Arthritis Rheumatol 71:109–120
Lang T, Lee JPW, Elgass K et al (2018) Macrophage migration inhibitory factor is required for NLRP3 inflammasome activation. Nat Commun 9:1–15
Dinarello CA (2006) Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr 83:447S-455S
Lee W-W, Kang SW, Choi J et al (2010) Regulating human Th17 cells via differential expression of IL-1 receptor. Blood J Am Soc Hematol 115:530–540
Martinon F, Pétrilli V, Mayor A et al (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241
Kolly L, Busso N, Palmer G et al (2010) Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology 129:178–185
Kahlenberg MM, Kang I (2020) The clinicopathologic significance of inflammasome activation in autoimmune diseases: inflammasome and autoimmune diseases. Arthritis Rheumatol 72:386. https://doi.org/10.1002/art.41127
Li Y, Shen Y, Jin K et al (2019) The DNA repair nuclease MRE11A functions as a mitochondrial protector and prevents T cell pyroptosis and tissue inflammation. Cell Metab 30:477–492
Zhao C, Gu Y, Zeng X, Wang J (2018) NLRP3 inflammasome regulates Th17 differentiation in rheumatoid arthritis. Clin Immunol 197:154–160
Cronstein BN, Sitkovsky M (2016) Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat Publ Gr. https://doi.org/10.1038/nrrheum.2016.178
Crofford LJ (2013) Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther 15:S2
Schett G, Emery P, Tanaka Y et al (2016) Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis 75:1428–1437. https://doi.org/10.1136/annrheumdis-2016-209201
Gerards AH, de Lathouder S, de Groot ER et al (2003) Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology 42:1189–1196. https://doi.org/10.1093/rheumatology/keg323
Brown PM, Pratt AG, Isaacs JD (2016) Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol 12:731–742. https://doi.org/10.1038/nrrheum.2016.175
Kerschbaumer A, Sepriano A, Smolen JS et al (2020) Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 79:S744–S759. https://doi.org/10.1136/annrheumdis-2019-216656
Goh L, Jewell T, Laversuch C, Samanta A (2013) A systematic review of the influence of anti-TNF on infection rates in patients with rheumatoid arthritis. Rev Bras Reumatol 53:501–515. https://doi.org/10.1016/j.rbre.2012.12.001
Bijlsma JWJ, Jacobs JWG (2008) Glucocorticoid chronotherapy in rheumatoid arthritis. Lancet 371:183
Mundell L, Lindemann R, Douglas J (2017) Monitoring long-term oral corticosteroids. BMJ Open Qual 6:e000209. https://doi.org/10.1136/bmjoq-2017-000209
Baschant U, Lane NE, Tuckermann J (2012) The multiple facets of glucocorticoid action in rheumatoid arthritis. Nat Rev Rheumatol 8:645–655. https://doi.org/10.1038/nrrheum.2012.166
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79:629–661
Manohar R (2013) Rheumatoid arthritis: ayurvedic perspectives. ASL Musculoskelet Dis 1:1. https://doi.org/10.4103/0000-1112.111926
Jaiswal YS, Williams LL (2016) Journal of traditional and complementary medicine review article a glimpse of ayurveda e the forgotten history and principles of Indian traditional medicine. J Tradit Chin Med Sci. https://doi.org/10.1016/j.jtcme.2016.02.002
Patwardhan B, Warude D, Pushpangadan P, Bhatt N (2005) Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Altern Med 2:465–473. https://doi.org/10.1093/ecam/neh140
Balkrishna A, Sakat SS, Joshi K et al (2019) Anti-inflammatory and anti-arthritic efficacies of an Indian traditional herbo-mineral medicine “Divya Amvatari Ras” in collagen antibody-induced arthritis (CAIA) mouse model through modulation of IL-6/IL-1β/TNF-α/NFκB signaling. Front Pharmacol 10:1–19. https://doi.org/10.3389/fphar.2019.00659
Panche AN, Diwan AD, Chandra SR (2016) Flavonoids: an overview. J Nutr Sci 5:e47
Hughes SD, Ketheesan N, Haleagrahara N (2017) The therapeutic potential of plant flavonoids on rheumatoid arthritis. Crit Rev Food Sci Nutr 57:3601–3613
Kelepouri D, Mavropoulos A, Bogdanos DP, Sakkas LI (2018) The role of flavonoids in inhibiting Th17 responses in inflammatory arthritis. J Immunol Res. https://doi.org/10.1155/2018/9324357
Wang Y, ling, Gao J mei, Xing LZ, (2016) Therapeutic potential of Oroxylin A in rheumatoid arthritis. Int Immunopharmacol 40:294–299. https://doi.org/10.1016/j.intimp.2016.09.006
Nakamura K, Matsuoka H, Nakashima S et al (2015) Oral administration of apple condensed tannins delays rheumatoid arthritis development in mice via downregulation of T helper 17 (Th17) cell responses. Mol Nutr Food Res 59:1406–1410
Carullo G, Cappello AR, Frattaruolo L et al (2017) Quercetin and derivatives: useful tools in inflammation and pain management. Future Med Chem 9:79–93
Hybertson BM, Gao B, Bose SK, McCord JM (2011) Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Asp Med 32:234–246
Guazelli CFS, Staurengo-Ferrari L, Zarpelon AC et al (2018) Quercetin attenuates zymosan-induced arthritis in mice. Biomed Pharmacother 102:175–184. https://doi.org/10.1016/j.biopha.2018.03.057
Pope RM (2002) Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat Rev Immunol 2:527–535
Wang G, Wu Y, Zhu Y (2018) Mechanism of MALAT1 preventing apoptosis of vascular endothelial cells induced by oxygen-glucose deficiency and reoxidation. Artif Cells Nanomed Biotechnol 46:798–805. https://doi.org/10.1080/21691401.2018.1436065
Pan F, Zhu L, Lv H, Pei C (2016) Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med 38:1507–1514. https://doi.org/10.3892/ijmm.2016.2755
Yuan K, Zhu Q, Lu Q et al (2020) Quercetin alleviates rheumatoid arthritis by inhibiting neutrophil inflammatory activities. J Nutr Biochem 84:108454. https://doi.org/10.1016/j.jnutbio.2020.108454
Ali F, Naz F, Jyoti S et al (2017) Health functionality of apigenin: a review Health functionality of apigenin: a review. Int J Food Prop 20:1197–1238. https://doi.org/10.1080/10942912.2016.1207188
Salehi B, Venditti A, Sharifi-Rad M et al (2019) The therapeutic potential of Apigenin. Int J Mol Sci. https://doi.org/10.3390/ijms20061305
Dosunmu A (2018) Apigenin and apigeninidin isolates from the Sorghum bicolor leaf targets inflammation via cyclo-oxygenase-2 and prostaglandin-E 2 blockade. Int J Rheum Dis 21:1487–1495. https://doi.org/10.1111/1756-185X.13355
Khan S, Greenberg JD, Bhardwaj N (2009) Dendritic cells as targets for therapy in rheumatoid arthritis. Nat Rev Rheumatol 5:566–571
Li X, Han Y, Zhou Q et al (2016) Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J Cell Mol Med 20:170–180
Li Y, Yang B, Bai J et al (2019) International immunopharmacology the roles of synovial hyperplasia, angiogenesis and osteoclastogenesis in the protective effect of apigenin on collagen-induced arthritis. Int Immunopharmacol 73:362–369. https://doi.org/10.1016/j.intimp.2019.05.024
Ganeshpurkar A, Saluja A (2019) The pharmacological potential of hesperidin. Indian J Biochem Biophys 56:287–300
Ahmad S, Alam K, Hossain MM et al (2016) Anti-arthritogenic and cardioprotective action of hesperidin and daidzein in collagen-induced rheumatoid arthritis. Mol Cell Biochem 423:115–127. https://doi.org/10.1007/s11010-016-2830-y
Matsuda S, Nakanishi A, Wada Y, Kitagishi Y (2013) Roles of PI3K/AKT/PTEN pathway as a target for harmaceutical therapy. Open Med Chem J 7:23
Qi W, Lin C, Fan K et al (2019) Hesperidin inhibits synovial cell inflammation and macrophage polarization through suppression of the PI3K/AKT pathway in complete Freund’s adjuvant-induced arthritis in mice. Chem Biol Interact 306:19–28. https://doi.org/10.1016/j.cbi.2019.04.002
Cici D, Corrado A, Rotondo C, Cantatore FP (2019) Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int J Mol Sci 20:5552
Deshmukh A, Arfuso F, Newsholme P, Dharmarajan A (2019) Epigenetic demethylation of sFRPs, with emphasis on sFRP4 activation, leading to Wnt signalling suppression and histone modifications in breast, prostate, and ovary cancer stem cells. Int J Biochem Cell Biol 109:23–32
Galli LM, Barnes T, Cheng T et al (2006) Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev Dyn an Off Publ Am Assoc Anat 235:681–690
Khan MA, Ahmed RS, Chandra N et al (2018) In vivo, extract from withania somnifera root ameliorates arthritis via regulation of key immune mediators of inflammation in experimental model of arthritis. Antiinflamm Antiallergy Agents Med Chem 18:55–70. https://doi.org/10.2174/1871523017666181116092934
Giri KR (2016) Comparative study of anti-inflammatory activity of Withania somnifera (Ashwagandha) with hydrocortisone in experimental animals (Albino rats). J Med Plants Stud 4:78–83
Ludwiczuk A, Skalicka-Woźniak K, Georgiev MI (2017) Terpenoids. In: Pharmacognosy. Elsevier, pp 233–266
Las Heras B, Rodriguez B, Bosca L, Villar AM (2003) Terpenoids: sources, structure elucidation and therapeutic potential in inflammation. Curr Top Med Chem 3:171–185
Babalola IT, Shode FO (2013) Ubiquitous ursolic acid: a potential pentacyclic triterpene natural product. J Pharmacogn Phytochem 2:214–222
Kang S-Y, Yoon S-Y, Roh D-H et al (2008) The anti-arthritic effect of ursolic acid on zymosan-induced acute inflammation and adjuvant-induced chronic arthritis models. J Pharm Pharmacol 60:1347–1354. https://doi.org/10.1211/jpp/60.10.0011
Kim EY, Sudini K, Singh AK et al (2018) Ursolic acid facilitates apoptosis in rheumatoid arthritis synovial fibroblasts by inducing SP1-mediated Noxa expression and proteasomal degradation of Mcl-1. FASEB J 32:6174–6185. https://doi.org/10.1096/fj.201800425R
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev cancer 9:798–809
Xu ZS, Zhang HX, Li WW et al (2019) FAM64A positively regulates STAT3 activity to promote Th17 differentiation and colitisassociated carcinogenesis. Proc Natl Acad Sci USA 116:10447–10452. https://doi.org/10.1073/pnas.1814336116
Jetten AM, Takeda Y, Slominski A, Kang HS (2018) Retinoic acid-related Orphan Receptor γ (RORγ): connecting sterol metabolism to regulation of the immune system and autoimmune disease. Curr Opin Toxicol 8:66–80
Xu T, Wang X, Zhong B et al (2011) Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORγt protein. J Biol Chem 286:22707–22710. https://doi.org/10.1074/jbc.C111.250407
Krause A, Scaletta N, Ji J, Ivashkiv L (2002) Rheumatoid arthritis synoviocyte survival is dependent on Stat3. J Immunol 169:6610–6616. https://doi.org/10.4049/jimmunol.169.11.6610
Gupta S, Mishra KP, Kumar B et al (2020) Andrographolide attenuates complete freund’s adjuvant induced arthritis via suppression of inflammatory mediators and pro-inflammatory cytokines. J Ethnopharmacol 261:113022. https://doi.org/10.1016/j.jep.2020.113022
Gupta S, Mishra KP, Singh SB, Ganju L (2018) Inhibitory effects of andrographolide on activated macrophages and adjuvant-induced arthritis. Inflammopharmacology 26:447–456. https://doi.org/10.1007/s10787-017-0375-7
Ha J, Choi H-S, Lee Y et al (2010) CXC chemokine ligand 2 induced by receptor activator of NF-κB ligand enhances osteoclastogenesis. J Immunol 184:4717–4724. https://doi.org/10.4049/jimmunol.0902444
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191:677–691. https://doi.org/10.1083/jcb.201006052
Strzepa A, Pritchard KA, Dittel BN (2017) Myeloperoxidase: a new player in autoimmunity. Cell Immunol 317:1–8. https://doi.org/10.1016/j.cellimm.2017.05.002
O’Neil LJ, Kaplan MJ (2019) Neutrophils in rheumatoid arthritis: breaking immune tolerance and fueling disease. Trends Mol Med 25:215–227. https://doi.org/10.1016/j.molmed.2018.12.008
Luo S, Li H, Liu J et al (2020) Andrographolide ameliorates oxidative stress, inflammation and histological outcome in complete Freund’s adjuvant-induced arthritis. Chem Biol Interact. https://doi.org/10.1016/j.cbi.2020.108984
Koushik S, Joshi N, Nagaraju S et al (2017) PAD4: pathophysiology, current therapeutics and future perspective in rheumatoid arthritis. Expert Opin Ther Targets 21:433–447
Li X, Yuan K, Zhu Q et al (2019) Andrographolide ameliorates rheumatoid arthritis by regulating the apoptosis–NETosis balance of neutrophils. Int J Mol Sci. https://doi.org/10.3390/ijms20205035
Shen ZT, Sigalov AB (2017) Rationally designed ligand-independent peptide inhibitors of TREM-1 ameliorate collagen-induced arthritis. J Cell Mol Med 21:2524–2534. https://doi.org/10.1111/jcmm.13173
Fan D, He X, Bian Y et al (2016) Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis. Int J Mol Sci 17:1–14. https://doi.org/10.3390/ijms17040498
Wright HL, Moots RJ, Bucknall RC, Edwards SW (2010) Neutrophil function in inflammation and inflammatory diseases. Rheumatology 49:1618–1631
Huang G, Yuan K, Zhu Q et al (2018) Triptolide inhibits the inflammatory activities of neutrophils to ameliorate chronic arthritis. Mol Immunol 101:210–220. https://doi.org/10.1016/j.molimm.2018.06.012
Wang S, Liu Z, Wang J et al (2019) The triptolide-induced apoptosis of osteoclast precursor by degradation of cIAP2 and treatment of rheumatoid arthritis of TNF-transgenic mice. Phyther Res 33:342–349. https://doi.org/10.1002/ptr.6224
Gong Y, Huang X, Di Wang ML, Liu Z (2017) Triptolide protects bone against destruction by targeting RANKL-mediated ERK/AKT signalling pathway in the collagen-induced rheumatoid arthritis. Biomed Res 28
Corradini E, Foglia P, Giansanti P et al (2011) Flavonoids: chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat Prod Res 25:469–495
Rizzato G, Scalabrin E, Radaelli M et al (2017) A new exploration of licorice metabolome. Food Chem 221:959–968
Hoxha M (2018) A systematic review on the role of eicosanoid pathways in rheumatoid arthritis. Adv Med Sci 63:22–29. https://doi.org/10.1016/j.advms.2017.06.004
Abbasi M, Mousavi MJ, Jamalzehi S et al (2019) Strategies toward rheumatoid arthritis therapy; the old and the new. J Cell Physiol 234:10018–10031. https://doi.org/10.1002/jcp.27860
Xie C, Li X, Wu J et al (2015) Anti-inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/arachidonic acid pathway. Inflammation 38:1639–1648. https://doi.org/10.1007/s10753-015-0140-2
Huang QC, Wang MJ, Chen XM et al (2016) Can active components of licorice, glycyrrhizin and glycyrrhetinic acid, lick rheumatoid arthritis? Oncotarget 7:1193–1202. https://doi.org/10.18632/oncotarget.6200
Andersson U, Harris HE (2010) The role of HMGB1 in the pathogenesis of rheumatic disease. Biochim Biophys Acta Gene Regul Mech 1799:141–148. https://doi.org/10.1016/j.bbagrm.2009.11.003
Wang WJ, Yin SJ, Rong RQ (2015) PKR and HMGB1 expression and function in rheumatoid arthritis. Genet Mol Res 14:17864–17870
Kato M, Ospelt C, Gay RE et al (2014) Dual role of autophagy in stress-induced cell death in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol 66:40–48
Shafik NM, El-Esawy RO, Mohamed DA et al (2019) Regenerative effects of glycyrrhizin and/or platelet rich plasma on type-II collagen induced arthritis: targeting autophay machinery markers, inflammation and oxidative stress. Arch Biochem Biophys 675:108095. https://doi.org/10.1016/j.abb.2019.108095
Liang B, Guo X-L, Jin J et al (2015) Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J Gastroenterol 21:5271–5280. https://doi.org/10.3748/wjg.v21.i17.5271
Zhou S, Liu G, Si Z et al (2020) Glycyrrhizin, an HMGB1 inhibitor, suppresses interleukin-1β-induced inflammatory responses in chondrocytes from patients with osteoarthritis. Cartilage. https://doi.org/10.1177/1947603520934858
Dean M, Murphy BT, Burdette JE (2017) Phytosteroids beyond estrogens: regulators of reproductive and endocrine function in natural products. Mol Cell Endocrinol 442:98–105
Khanna D, Sethi G, Ahn KS et al (2007) Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol 7:344–351
Lee Y-R, Lee J-H, Noh E-M et al (2008) Guggulsterone blocks IL-1β-mediated inflammatory responses by suppressing NF-κB activation in fibroblast-like synoviocytes. Life Sci 82:1203–1209
Boyce BF, Xing L (2008) Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys 473:139–146. https://doi.org/10.1016/j.abb.2008.03.018
Ichikawa H, Aggarwal BB (2006) Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-κB ligand and by tumor cells by suppressing nuclear factor-κB activation. Clin cancer Res 12:662–668
Tiwari P, Nayak P, Prusty SK, Sahu PK (2018) Phytochemistry and pharmacology of Tinospora cordifolia: a review. Syst Rev Pharm 9:70–78
Hui W, Dai Y (2020) Therapeutic potential of aryl hydrocarbon receptor ligands derived from natural products in rheumatoid arthritis. Basic Clin Pharmacol Toxicol 126:469–474. https://doi.org/10.1111/bcpt.13372
Ishihara Y, Kado SY, Hoeper C et al (2019) Role of NF-kB RelB in aryl hydrocarbon receptor-mediated ligand specific effects. Int J Mol Sci 20:2652
Tong B, Yuan X, Dou Y et al (2016) Norisoboldine, an isoquinoline alkaloid, acts as an aryl hydrocarbon receptor ligand to induce intestinal Treg cells and thereby attenuate arthritis. Int J Biochem Cell Biol 75:63–73. https://doi.org/10.1016/j.biocel.2016.03.014
Øvrevik J, Låg M, Lecureur V et al (2014) AhR and Arnt differentially regulate NF-κB signaling and chemokine responses in human bronchial epithelial cells. Cell Commun Signal 12:1–17. https://doi.org/10.1186/s12964-014-0048-8
Wei ZF, Lv Q, Xia Y et al (2015) Norisoboldine, an anti-arthritis alkaloid isolated from radix linderae, attenuates osteoclast differentiation and inflammatory bone erosion in an aryl hydrocarbon receptor-dependent manner. Int J Biol Sci 11:1113–1126. https://doi.org/10.7150/ijbs.12152
Luo Y, Wei Z, Chou G et al (2014) Norisoboldine induces apoptosis of fibroblast-like synoviocytes from adjuvant-induced arthritis rats. Int Immunopharmacol 20:110–116. https://doi.org/10.1016/j.intimp.2014.02.023
Vorrink SU, Domann FE (2014) Regulatory crosstalk and interference between the and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node. Chem Biol Interact 218:82–88. https://doi.org/10.1016/j.cbi.2014.05.001
Tong B, Dou Y, Wang T et al (2015) Norisoboldine ameliorates collagen-induced arthritis through regulating the balance between Th17 and regulatory T cells in gut-associated lymphoid tissues. Toxicol Appl Pharmacol 282:90–99. https://doi.org/10.1016/j.taap.2014.11.008
Shen P, Jiao Y, Miao L, Amir JC (2020) Immunomodulatory effects of berberine on the inflamed joint reveal new therapeutic targets for rheumatoid arthritis management. J Cell Mol Med 24:12234–12245. https://doi.org/10.1111/jcmm.15803
Chander V, Aswal JS, Dobhal R, Uniyal DP (2017) A review on pharmacological potential of Berberine; an active component of Himalayan Berberis aristata. J Phytopharmacol 6(1):53–58
Vomero M, Barbati C, Colasanti T et al (2018) Autophagy and rheumatoid arthritis: current knowledges and future perspectives. Front Immunol 9:1577
Dinesh P, Rasool M (2019) Berberine mitigates IL - 21 / IL - 21R mediated autophagic influx in fibroblast—like synoviocytes and regulates Th17/Treg imbalance in rheumatoid arthritis. Apoptosis 24:644–661. https://doi.org/10.1007/s10495-019-01548-6
Zhou J, Yu Y, Yang X et al (2019) Berberine attenuates arthritis in adjuvant-induced arthritic rats associated with regulating polarization of macrophages through AMPK/NF-к B pathway. Eur J Pharmacol 852:179–188. https://doi.org/10.1016/j.ejphar.2019.02.036
Acknowledgements
The authors would like to acknowledge the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patidar, V., Shah, S., Kumar, R. et al. A molecular insight of inflammatory cascades in rheumatoid arthritis and anti-arthritic potential of phytoconstituents. Mol Biol Rep 49, 2375–2391 (2022). https://doi.org/10.1007/s11033-021-06986-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06986-7