Abstract
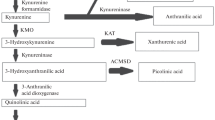
Viral diseases of the brain may induce changes in neurotransmitter synthesis and metabolism. In experimental herpes simplex encephalitis, brain serotonin is reduced, whilst it’s major metabolite, 5-hydroxyindole acetic acid and turnover is increased. It is well established that reduced levels of brain monoamines, serotonin and norepinephrine may contribute to the symptoms of clinical depression, which raises the possibility that this condition is prevalent in herpes simplex encephalitis. An inverse relationship exists between liver tryptophan-2,3-dioxygenase activity and brain serotonin levels and there is an interdependency between serotonin and norepinephrine levels. The aim of this study is to determine the effect of acyclovir, an antiviral used in the treatment of herpes simplex encephalitis, on rat liver tryptophan-2,3-dioxygenase activity in vitro and in vivo as well as on rat forebrain serotonin, 5-hydroxyindole acetic acid and norepinephrine levels. The results show that acyclovir inhibits tryptophan-2,3-dioxygenase activity in vitro and in vivo, with a concomitant rise in serotonin and 5-hydroxyindole acetic acid levels. However, acyclovir reduces the turnover of serotonin to 5-hydroxyindole acetic acid, without any effect on norepinephrine levels. It appears that acyclovir may have the potential to reduce the clinical symptoms of depression in herpes simplex encephalitis. However, a greater turnover of serotonin to 5-hydroxyindole acetic acid could possibly be masked by conversion of serotonin to 5-hydroxytryptophol, which needs to be investigated further.


Similar content being viewed by others
References
Badawy AAB, Evans M (1975) The regulation of rat liver tryptophan pyrrolase by its cofactor haeme. J Biochem 150:511–520
Badawy AAB, Evans M (1981) Inhibition of rat liver tryptophan pyrrolase activity and elevation of brain tryptophan concentration by administration of anti-depressants. Biochem Pharmacol 30:1211–1216
Bender DA (1983) Biochemistry of tryptophan in health and disease. Mol Aspects Med 6:101–197
Daya S, Nonaka KO, Buzzell GR, Reiter RJ (1989) Haeme precursor 5-aminolevulinic acid alters brain tryptophan and serotonin levels without changing pineal serotonin and melatonin concentrations. Journal Neurosci Res 23:304–309
Delgado PL, Charney RS, Price LH, Aghajanian GK, Randis H, Henninger GR (1990) Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry 47:411–418
Gibbons RD, Davis JM (1986) Consistent evidence for a biological subtype of depression characterised by low CSF monoamine levels. Acta Psychiatr Scand 74:8–12
Groff JL, Gropper SS (1999) Advanced Nutrition and Human Metabolism, 3rd edn. Wassworth/Thomson Learning, Belmont
Kopin IJ (1981) Mode of action of antidepressants and central stimulants. In: van Praag HM, Lader MH, Rafaelsen OJ, Sachar EJ (eds) Handbook of biological psychiatry: Part IV. Marcel Dekker, New York
Lovenberg W, Weissbach H, Udenfriend A (1962) Aromatic L-amino acid decarboxylase. J Biol Chem 237:89–93
Lovenberg W, Jequier E, Sjoerdsma A (1967) Tryptophan hydroxylation. Measurement in pineal gland, brain stem and carcinoid tumour. Science 155:217–219
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lycke E, Roos B-E (1972) The monoamine metabolism in viral encephalides of the mouse II. Turnover of monoamines in mice infected with herpes simplex virus. Brain Res 44:603–613
McTavish SF, Cowen PJ, Sharp T (1999) Effect of a tyrosine-free amino acid mixture on regional brain catecholamine synthesis and release. Psychopharmacologia 141:182–188
Müller AC, Maharaj H, Maharaj DS, Daya S (2005) Acyclovir protects against quinolinic acid-induced oxidative neurotoxicity. J Pharm Pharmacol 57:883–888
Müller AC, Dairam A, Limson JL, Daya S (2007) Mechanisms by which acyclovir reduces the oxidative neurotoxicity and biosynthesis of quinolinic acid. Life Sci 80:918–925
Muralikrishnan D, Mohanakumar KP (1998) Neuroprotection by bromocriptine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in mice. FASEB J 12:905–912
Neeley SP, Cross AJ, Crow TJ, Johnson JA, Taylor GR (1985) Herpes simplex virus encephalitis: Neuroanatomical and neurochemical selectivity. J Neurol Sci 71:325–337
Päivärinta MA, Marttila RJ, Lonnberg P, Rinne UK (1993) Decreased raphe serotonin in rabbits with experimental herpes simplex encephalitis. Neurosci Lett 156:1–4
Reader TA, Ase AR, Le Marec N, Lalonde R (2000) Effects of buspirone on brain indoleamines and catecholamines in wild-type mice and Lurcher mutants. Eur J Pharmacology 398:41–51
Richards DM, Carmine AA, Brogden RN, Heel RC, Speight TM, Avery GS (1983) Acyclovir: a review of its pharmacodynamic properties and therapeutic efficacy. Drugs 26:378–438
Schaechter JD, Wurtman RJ (1989) Tryptophan availability modulates serotonin release from rat hypothalamic slices. J Neurochem 53:1925–1935
Shein HM, Wurtman RJ (1971) Stimulation of [14C] tryptophan 5-hydroxylation by norepinephrine and dibutyryl adenosine 3′, 5′ monophosphate in rat pineal organ cultures. Life Sci 10:935–940
Uchida K, Usami M, Bandow H, Harada I (1992) Characteristics of substrates and inhibitors in binding to rat liver L-tryptophan-2,3-dioxygenase: a Fourier transform infrared and kinetic study. Biochim Biophys Acta 1121:153–159
Van Praag HM (1982a) Depression, suicide and serotonin metabolism in the brain. In: Post RM, Ballenger JC (eds) The neurobiology of manic depressive illness. Williams and Wilkins, Baltimore
Van Praag HM (1982b) Neurotransmitters and CNS disease. Lancet 2:1259–1264
Wurtman RJ, Larin F, Axelrod J, Shein HM, Rosasco K (1968) Formation of melatonin and 5-hydroxy-indole acetic acid from 14C-tryptophan by rat pineal glands in organ culture. Nature 217:953
Xing-Ming Li S, Perry KW, Wong DT (2002) Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of rats. Neuropharmacology 42:181–190
Zar JH (1974) Biostatistical Analysis. Prentice Hall, Engelwood Cliffs
Acknowledgements
The authors thank Sally and Dave Morley for their technical assistance. They are grateful to the National Research Foundation (ACM and SD), The South African Medical Research Council (SD) and the Andrew Mellon Foundation, Rhodes University (ACM) for financial support
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, A.C., Daya, S. Acyclovir inhibits rat liver tryptophan-2,3-dioxygenase and induces a concomitant rise in brain serotonin and 5-hydroxyindole acetic acid levels. Metab Brain Dis 23, 351–360 (2008). https://doi.org/10.1007/s11011-008-9095-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-008-9095-4