Abstract
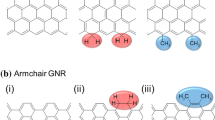
Determination of the number of bromo groups and brominated positions of edges on carbon materials is essential for selective functionalization because the brominated positions influence their electronic structures. However, the determination is challenging because the analysis of introduced bromo groups on carbon materials is conventionally limited to quantitative analysis by X-ray photoelectron spectroscopy. In this work, infrared spectra (IR) of brominated aromatic compounds were analyzed experimentally and theoretically using reference aromatic compounds to verify that the calculated peak positions can be used to estimate the experimental peak positions of brominated aromatic compounds. IR spectra of brominated graphene nanoribbons (GNRs) with zigzag, armchair, and other edges were simulated as one of the carbon materials with clear edge structures. Their characteristic peak positions and tendencies of shifts were explained from the point of view of Mulliken charge, C–H and C=C length, and orbital hybridization. As the number of Br on GNRs increased, the peak positions basically shifted further, indicating that the density of Br and introduced positions of Br can be estimated from IR spectra. The detailed assignments obtained in this work will lead to selective functionalization of carbon materials.















Similar content being viewed by others
References
Castro AHN, Guinea F, Peres NMR, Novoselov KS, Geim AK (2009) The electronic properties of graphene. Rev Mod Phys 81:109–162
Candini A, Martini L, Chen Z, Mishra N, Convertino D, Coletti C et al (2017) High photoresponsivity in graphene nanoribbon field-effect transistor devices contacted with graphene electrodes. J Phys Chem C 121:10620–10625
Chen YC, de Oteyza DG, Pedramrazi Z, Chen C, Fischer FR, Crommie MF (2013) Tuning the band gap of graphene nanoribbons synthesized from molecular precursors. ACS Nano 7:6123–6128
Magda GZ, Jin X, Hagymási I, Vancsó P, Osváth Z, Nemes-Incze P et al (2014) Room-temperature magnetic order on zigzag edges of narrow graphene nanoribbons. Nature 514:608–611
Merino-Díez N, Garcia-Lekue A, Carbonell-Sanromà E, Li J, Corso M, Colazzo L et al (2017) Width-dependent band gap in armchair graphene nanoribbons reveals Fermi level pinning on Au(111). ACS Nano 11:11661–11668
Wagner P, Ewels CP, Adjizian JJ, Magaud L, Pochet P, Roche S et al (2013) Band gap engineering via edge-functionalization of graphene nanoribbons. J Phys Chem C 117:26790–26796
Zheng H, Zheng JJ, He L, Zhao X (2014) Unique configuration of a nitrogen-doped graphene nanoribbon: potential applications to semiconductor and hydrogen fuel cell. J Phys Chem C 118:24723–24729
Saikia I, Borah AJ, Phukan P (2016) Use of bromine and bromo-organic compounds in organic synthesis. Chem Rev 116:6837–7042
Tasker SZ, Standley EA, Jamison TF (2014) Recent advances in homogeneous nickel catalysis. Nature 509:299–309
Miyaura N, Yamada K, Suzuki A (1979) A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett 20:3437–3440
Miyaura N, Suzuki A (1995) Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev 95:2457–2483
Cai J, Ruffieux P, Jaafar R, Bieri M, Braun T, Blankenburg S et al (2010) Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 46:470–473
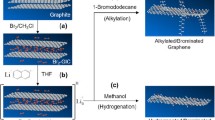
Kim J, Yamada Y, Fujita R, Sato S (2015) Bromination of graphene with pentagonal, hexagonal zigzag and armchair, and heptagonal edges. J Mater Sci 50:5183–5190. https://doi.org/10.1007/s10853-015-9066-1
Friedrich JF, Wettmarshausen S, Hanelt S, Mach R, Mix R, Zeynalov E et al (2010) Plasma-chemical Bromination of graphitic materials and its use for subsequent functionalization and grafting of organic molecules. Carbon 48:3884–3894
Hanelt S, Friedrich JF, Orts-Gil G, Meyer-Plath A (2012) Study of Lewis acid catalyzed chemical bromination and bromoalkylation of multi-walled carbon nanotubes. Carbon 50:1373–1385
Mansour AE, Dey S, Amassian A (2015) Bromination of graphene: a new route to making high performance transparent conducting electrodes with low optical losses. ACS Appl Mater Interfaces 7:17692–17699
Bulusheva LG, Okotrub AV, Flahaut E, Asanov IP, Gevko PN, Koroteev VO et al (2012) Bromination of double-walled carbon nanotubes. Chem Mater 24:2708–2715
Sasaki T, Yamada Y, Sato S (2018) Quantitative analysis of zigzag and armchair edges on carbon materials with/without pentagons using infrared spectroscopy. Anal Chem 90:10724–10731
Yamada Y, Gohda S, Abe K, Togo T, Shimano N, Sasaki T et al (2017) Carbon materials with controlled edge structures. Carbon 122:694–701
Yamada Y, Kawai M, Yorimitsu H, Otsuka S, Takanashi M, Sato S (2018) Carbon materials with zigzag and armchair edges. ACS Appl Mater Interfaces 10:40710–40739
Senda T, Yamada Y, Morimoto M, Nono N, Sogabe T, Kubo S et al (2019) Analyses of oxidation process for isotropic pitch-based carbon fiber using model compounds. Carbon 142:311–326
Tommasini M, Lucotti A, Alfè M, Ciajolo A, Zerbi G (2016) Fingerprints of polycyclic aromatic hydrocarbons (PAHs) in infrared absorption spectroscopy. Spectrochim Acta A 152:134–148
Russo C, Stanzione F, Tregrossi A, Ciajolo A (2014) Infrared spectroscopy of some carbon-based materials relevant in combustion: qualitative and quantitative analysis of hydrogen. Carbon 74:127–138
Centrone A, Brambilla L, Renouard T, Gherghel L, Mathis C, Müllen K et al (2005) Structure of new carbonaceous materials: the role of vibrational spectroscopy. Carbon 43:1593–1609
Zheng J, Liu HT, Wu B, Di CA, Guo YL, Wu T et al (2012) Production of graphite chloride and bromide using microwave sparks. Sci Rep 2:662
Jankovský O, Šimek P, Klimová K, Sedmidubský D, Matějková S, Pumera M et al (2014) Towards graphene bromide: bromination of graphite oxide. Nanoscale 6:6065–6074
He J, Bao F, Yan S, Weng F, Ma R, Liu Y, Ding H (2017) Soluble fluorene–benzothiadiazole polymer-grafted graphene for photovoltaic devices. RSC Adv 7:35950–35956
Yao Y, Gao J, Bao F, Jiang S, Zhang X, Ma R (2015) Covalent functionalization of graphene with polythiophene through a Suzuki coupling reaction. RSC Adv 5:42754–42761
Mooney EF (1963) The infrared spectra of chloro- and bromobenzene derivatives-I anisoles and phenetoles. Spectrochim Acta 19:877–887
Fuente E, Menéndez A, Díez MA, Suárez D, Montes-Morán MA (2003) Infrared spectroscopy of carbon materials: a quantum chemical study of model compounds. J Phys Chem B 107:6350–6359
Karlický F, Datta KKR, Otyepka M, Zbořil R (2013) Halogenated graphenes: rapidly growing family of graphene derivatives. ACS Nano 7:6434–6464
Palafox AP (2017) Computational chemistry applied to vibrational spectroscopy: a tool for characterization of nucleic acid bases and some of their 5-substituted derivatives. Phys Sci Rev 20160132.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, et al. (2009) Gaussian 09, revision E.01; Gaussian, Inc., Wallingford CT.
Pein BC, Seong NH, Dlott DD (2010) Vibrational energy relaxation of liquid aryl-halides X-C6H5 (X = F, Cl, Br, I). J Phys Chem A 114:10500–10507
Larkin PJ (2011) Infrared and Raman spectroscopy principles and spectral interpretation. Elsevier Inc., Amsterdam, pp 10–132
Colthup NB, Daly LH, Wiberley SE (1990) Introduction to infrared and Raman spectroscopy. Academic Press, Massachusetts, pp 261–279
Yamada Y, Matsuo S, Abe K, Kubo S, Sato S (2016) Selective doping of nitrogen into carbon materials without catalysts. J Mater Sci 51:8900–8915. https://doi.org/10.1007/s10853-016-0142-y
Kim J, Lee N, Nodo M, Min YH, Noh SH, Kim N (2018) Distinguishing zigzag and armchair edges on graphene nanoribbons by X-ray photoelectron and Raman spectroscopies. ACS Omega 3:17789–17796
Eckmann A, Felten A, Mishchenko A, Britnell L, Krupke R, Novoselov KS et al (2012) Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett 12:3925–3930
Schaffer HE, Chance RR, Silbey RJ, Knoll K, Schrock RR (1991) Conjugation length dependence of Raman scattering in a series of linear polyenes: implications for polyacetylene. J Chem Phys 94:4161–4170
Yamada Y, Murota K, Fujita R, Kim J, Watanabe A, Nakamura M et al (2014) Subnanometer vacancy defects introduced on graphene by oxygen gas. J Am Chem Soc 136:2232–2235
McKean DC (1978) Individual CH bond strengths in simple organic compounds: effects of conformation and substitution. Chem Soc Rev 7:399–422
McKean DC (1984) New light on the stretching vibrations, lengths and strengths of CH, SiH and GeH bonds. J Mol Struct 113:251–266
Acknowledgements
This work was supported by Kondo Memorial Foundation in Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamada, Y., Masaki, S. & Sato, S. Brominated positions on graphene nanoribbon analyzed by infrared spectroscopy. J Mater Sci 55, 10522–10542 (2020). https://doi.org/10.1007/s10853-020-04786-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04786-1