Abstract
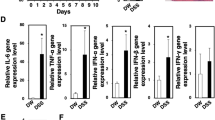
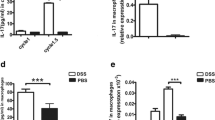
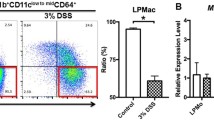
Crohn’s disease (CD) and ulcerative colitis (UC) are the most widely known types of inflammatory bowel diseases (IBD) and have been paid more attention due to their increasing incidence and a substantial increase in the risk of colorectal cancer (CRC). However, the phenotype and, more importantly, the function in the regulation of mucosal inflammation by different macrophages are poorly understood, even though macrophages constitute a major subset of intestinal myeloid cells. The results firstly showed that the subset of peritoneal CD11b+CD169+ macrophages increased and CCL22 expression level decreased significantly during the DSS-induced colitis. DSS-induced colitis was alleviated in CD169-DTR mice at least partially due to the deletion CD169+ macrophages. Moreover, the CCL22 expression level in peritoneal macrophages from CD169-DTR mice was much higher than that from WT mice with DSS-induced colitis. And, the cell-sorting result revealed that CD11b+CD169+ macrophage cells did not express CCL22 dominantly. Further experiment in vivo demonstrated that treatment with recombinant murine CCL22 (rmCCL22) ameliorated the clinical symptoms of DSS-induced colitis. All these data indicated that macrophage subset of CD11b+CD169+ from peritoneal cavity played critical role probably together with low levels of CCL22 in DSS-induced colitis.







Similar content being viewed by others
Abbreviations
- CD169:
-
Cluster of differentiation 169
- CCL22:
-
C-C motif chemokine 22
- DSS:
-
Dextran sulfate sodium
- DT:
-
Diphtheria toxin
- DTR:
-
Diphtheria toxin receptor
- Tregs:
-
Regulatory T cells
- UC:
-
Ulcerative colitis
- IBD:
-
Inflammatory bowel diseases.
Reference
Kilcoyne, A., J.L. Kaplan, and M.S. Gee. 2016. Inflammatory bowel disease imaging: Current practice and future directions. World Journal of Gastroenterology 22: 917–932.
Kaistha, A., and J. Levine. 2014. Inflammatory bowel disease: The classic gastrointestinal autoimmune disease. Current Problems in Pediatric and Adolescent Health Care 44: 328–334.
Itzkowitz, S.H., and X. Yio. 2004. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. American Journal of Physiology: Gastrointestinal and Liver Physiology 287: G7–17.
de Souza, H.S., and C. Fiocchi. 2016. Immunopathogenesis of IBD: Current state of the art. Nature Reviews. Gastroenterology & Hepatology 13: 13–27.
Davies, L.C., S.J. Jenkins, J.E. Allen, and P.R. Taylor. 2013. Tissue-resident macrophages. Nature Immunology 14: 986–995.
Gross, M., T.M. Salame, and S. Jung. 2015. Guardians of the Gut-Murine Intestinal Macrophages and Dendritic Cells. Frontiers in Immunology 6: 254.
Yona, S., K.W. Kim, Y. Wolf, A. Mildner, D. Varol, M. Breker, et al. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38: 79–91.
Asano, K., N. Takahashi, M. Ushiki, M. Monya, F. Aihara, E. Kuboki, et al. 2015. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nature Communications 6: 7802.
Martinez-Pomares, L., and S. Gordon. 2012. CD169+ macrophages at the crossroads of antigen presentation. Trends in Immunology 33: 66–70.
Asano, K., A. Nabeyama, Y. Miyake, C.H. Qiu, A. Kurita, M. Tomura, et al. 2011. CD169-positive macrophages dominate antitumor immunity by cross presenting dead cell-associated antigens. Immunity 34: 85–95.
Ravishankar, B., R. Shinde, H. Liu, K. Chaudhary, J. Bradley, H.P. Lemos, et al. 2014. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proceedings of the National Academy of Sciences of the United States of America 111: 4215–4220.
Hiemstra, I.H., M.R. Beijer, H. Veninga, K. Vrijland, E.G. Borg, B.J. Olivier, et al. 2014. The identification and development requirements of colonic CD169+ macrophages. Immunology: 142, 269–278.
Cassado Ados, A., M.R. D'Império Lima, and K.R. Bortoluci. 2015. Revisiting mouse peritoneal macrophages: heterogeneity, development, and function. Frontiers in Immunology 6: 225.
Geremia, A., P. Biancheri, P. Allan, G.R. Corazza, and A. Di Sabatino. 2014. Innate and adaptive immunity in inflammatory bowel disease. Autoimmunity Reviews 13: 3–10.
Wang, D., R.N. Dubois, and A. Richmond. 2009. The role of chemokines in intestinal inflammation and cancer. Current Opinion in Pharmacology 9: 688–696.
Zhang, J., J. Romero, A. Chan, J. Goss, S. Stucka, J. Cross, et al. 2015. Biarylsulfonamide CCR9 inhibitors for inflammatory bowel disease. Bioorganic & Medicinal Chemistry Letters 25: 3361–3364.
Evans-Marin, H.L., A.T. Cao, S. Yao, F. Chen, C. He, H. Liu, et al. 2015. Unexpected Regulatory Role of CCR9 in Regulatory T Cell Development. PloS One 10: e0134100.
Saito, Michiko, l Takao Iwawaki, Choji Taya, Hiromichi Yonekawa, Munehiro Noda, et al. Diphtheria toxin receptor–mediated conditional and targeted cell ablation in transgenic mice. Nature Biotechnology 19: 746–750.
Miyake, Y. l, K. Asano, H. Kaise, M. Uemura, M. Nakayama, and M. Tanaka. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. The Journal of Clinical Investigation 117: 2268–2278.
Saito, M., T. Iwawaki, C. Taya, H. Yonekawa, M. Noda, Y. Inui, et al. 2001. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nature Biotechnology 19: 746–750.
Herwald, H., and A. Egesten. 2013. Macrophages: past, present and future. Journal of Innate Immunity 5: 657–658.
Wynn, T.A., A. Chawla, and J.W. Pollard. 2013. Macrophage biology in development, homeostasis and disease. Nature 496: 445–455.
Ghosn, E.E., A.A. Cassado, G.R. Govoni, T. Fukuhara, Y. Yang, D.M. Monack, et al. 2010. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A107: 2568–2573.
Davies, L.C., M. Rosas, P.J. Smith, D.J. Fraser, S.A. Jones, and P.R. Taylor. 2011. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. European Journal of Immunology 41: 2155–2164.
Davies, L.C., M. Rosas, S.J. Jenkins, C.T. Liao, M.J. Scurr, F. Brombacher, et al. 2013. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nature Communications 4: 1886.
Okabe, Y., and R. Medzhitov. 2014. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157: 832–844.
Dahdah, A., G. Gautier, T. Attout, F. Fiore, E. Lebourdais, R. Msallam, et al. 2014. Mastcells aggravate sepsis by inhibiting peritoneal macrophage phagocytosis. The Journal of Clinical Investigation 124: 4577–4589.
Saunderson, S.C., A.C. Dunn, P.R. Crocker, and A.D. McLellan. 2014. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 123: 208–216.
Chávez-Galán, L., M.L. Olleros, D. Vesin, and I. Garcia. 2015. Much More than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Frontiers in Immunology 6: 263.
Ohnishi, K., M. Yamaguchi, C. Erdenebaatar, F. Saito, H. Tashiro, H. Katabuchi, et al. 2016. Prognostic significance of CD169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Science 107: 846–852.
Li, C., X. Luo, Y. Lin, X. Tang, L. Ling, L. Wang, et al. 2015. A Higher Frequency of CD14+ CD169+ Monocytes/Macrophages in Patients with Colorectal Cancer. PloS One 10: e0141817.
Saito, Y., K. Ohnishi, A. Miyashita, S. Nakahara, Y. Fujiwara, H. Horlad, et al. 2015. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunology Research 3: 1356–1363.
Kim, T.W., J.N. Seo, Y.H. Suh, H.J. Park, J.H. Kim, J.Y. Kim, et al. 2006. Involvement of lymphocytes in dextran sulfate sodium-induced experimental colitis. World Journal of Gastroenterology 12: 302–305.
Tlaskalová-Hogenová, H., L. Tucková, R. Stepánková, T. Hudcovic, L. Palová-Jelínková, H. Kozáková, et al. 2005. Involvement of innate immunity in the development of inflammatory and autoimmune diseases. Annals of the New York Academy of Sciences 1051: 787–798.
Hartnell, A., J. Steel, H. Turley, M. Jones, D.G. Jackson, and P.R. Crocker. 2001. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97 (1): 288–296.
Yoshie, O., and K. Matsushima. 2015. CCR4 and its ligands: from bench to bedside. International Immunology 27: 11–20.
Hao, S., X. Han, D. Wang, Y. Yang, Q. Li, X. Li, et al. 2016. Critical role of CCL22/CCR4 axis in the maintenance of immune homeostasis during apoptotic cell clearance by splenic CD8a+CD103+ DCs. Immunology 148: 174–186.
Owaga, E., R.H. Hsieh, B. Mugendi, S. Masuku, C.K. Shih, and J.S. Chang. 2015. Th17 Cells as Potential Probiotic Therapeutic Targets in Inflammatory Bowel Diseases. International Journal of Molecular Sciences 16: 20841–20858.
Gálvez, J. 2014. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm 2014: 928461.
Sartor, R.B. 2006. Mechanisms of disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepato 3: 390–407.
Zhang, Z., M. Zheng, J. Bindas, P. Schwarzenberger, and J.K. Kolls. 2006. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory Bowel Diseases 12: 382–388.
Monteleone, Ivan, Francesco Pallone, and Giovanni Monteleone. 2011. Th17-related cytokines: new players in the control of chronic intestinal inflammation. BMC Medicine 9: 122.
Sanchez-Munoz, F., A. Dominguez-Lopez, and J.K. Yamamoto-Furusho. 2008. Role of cytokines in inflammatory bowel disease. World Journal of Gastroenterology 14: 4280–4288.
Acknowledgments
The present work was supported by grants from the (NSFC), (No. 81202306), China Postdoctoral Science Foundation (201252M1343, 2013T60674). We would like to thank Dr. Yunxue Zhao and Ms. Limei Wang at Shandong University for FACS analysis. Qiu C. and Wang D. designed the study. Wang D., Li Q., Yang Y., Hao S., Han X., Song J., and Yin Y. performed experiments. Wang D., Li X, and Qiu C. analyzed the data. Qiu C. and Wang D. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest
Electronic supplementary material
ESM 1
(PPTX 266 kb)
Rights and permissions
About this article
Cite this article
Wang, D., Li, Q., Yang, Y. et al. Macrophage Subset Expressing CD169 in Peritoneal Cavity-Regulated Mucosal Inflammation Together with Lower Levels of CCL22. Inflammation 40, 1191–1203 (2017). https://doi.org/10.1007/s10753-017-0562-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-017-0562-0