Abstract
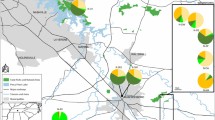
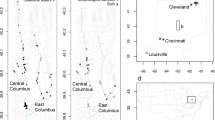
Restoration of habitat for endangered species often involves translocation of seeds or individuals from source populations to an area targeted for revegetation. Long-term persistence of a species is dependent on the maintenance of sufficient genetic variation within and among populations. Thus, knowledge and maintenance of genetic variability within rare or endangered species is essential for developing effective conservation and restoration strategies. Genetic monitoring of both natural and restored populations can provide an assessment of restoration protocol success in establishing populations that maintain levels of genetic diversity similar to those in natural populations. California’s vernal pools are home to many endangered plants, thus conservation and restoration are large components of their management. Lasthenia conjugens (Asteraceae) is a federally endangered self-incompatible vernal pool annual with gravity- dispersed seeds. Using the molecular technique of intersimple sequence repeats (ISSRs), this study assessed levels and patterns of genetic variability present within natural and restored populations of L. conjugens. At Travis Air Force Base near Fairfield, California, a vernal pool restoration project is underway. Genetic success of the ecologically based seeding protocol was examined through genetic monitoring of natural and restored populations over a three-year period. Genetic diversity remained constant across the three sampled generations. Diversity was also widely distributed across all populations. We conclude that the protocol used to establish restored populations was successful in capturing similar levels and patterns of genetic diversity to those seen within natural pools. This study also demonstrates how genetic markers can be used to inform conservation and restoration decisions.
Similar content being viewed by others
References
Baskin Y (1994) California’s ephemeral vernal pools may be a good model for speciation. BioScience 44: 384–388
Biosystems Analysis (1994). Vernal pool resources at Travis Air Force Base, Solano County, California: final report. Biosystems Analysis Inc., Tiburon, CA
Black C, Zedler PH (1998) An overview of 15 years of vernal pool restoration and construction activities in San Diego County, California. In: Witham CW, Bauder ET, Belk D, Ferren WRJ, Ornduff R (eds) Ecology, Conservation, and Management of Vernal Pool Ecosystems - Proceedings from a 1996 conference. California Native Plant Society, Sacramento, CA, pp. 195–205
Bussell JD (1999) The distribution of random amplified polymorphic DNA (RAPD) diversity amongst population of Isotoma petraea (Lobeliaceae). Mol. Ecol. 8: 775–789
Chan R (2001) A new section in the Goldfield genus Lasthenia (Compositae: Heliantheae sensu lato). Madrono 48: 38–39
Culley TM, Wolfe AD (2001) Population genetic structure of the cleistogamous plant species Viola pubescens Aiton (Violaceae), as indicated by allozyme and ISSR molecular markers. Heredity 86: 545–556
Dawson IK, Simons AH, Waugh R, Powell W (1995) Diversity and genetic differentiation among subpopulations of Gliricidia sepium revealed by PCR-based assays. Heredity 75: 10–18
Desrochers AM, Dodge B (2003) Phylogenetic relationships in Lasthenia (Heliantheae: Asteraceae) based on nuclear rDNA internal transcribed spacer (ITS) sequence data. Sys. Bot. 28: 208–215
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phyto. Bul. 19: 11–15
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distance among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131: 479–491
Falk DA, Holsinger KE (1991) Genetics and Conservation of Rare Plants. Oxford University Press, New York
Ferren WRJ, Hubbard DM, Wiseman S, Parikh AK, Gale N (1998) Review of ten years of vernal pool restoration and creation in Santa Barbara, California. In: Witham CW, Bauder ET, Belk D, Ferren WRJ, Ornduff R (eds) Ecology, conservation, and management of vernal pool ecosystems - Proceedings from a 1996 conference. California Native Plant Society, Sacramento, CA, pp. 206–216
Fischer M, Hock M, Paschke M (2003) Low genetic variation reduces cross-compatibility and offspring fitness in populations of a narrow endemic plant with a self-incompatibility system. Conserv. Genet. 4: 325–336
Fisher RA (1930) The fundamental theorem of natural selection. In: The Genetical Theory of Natural Selection, pp. 22–47. Clarendon Press, Oxford.
Fleishman E, Launer AE, Switky KR, Yandell U, Heywood J, Murphy DD (2001) Rules and exceptions in conservation genetics: genetic assessment of the endangered plant Cordylanthus palmatus and its implications for management planning. Biol. Conserv. 98: 45–53
Frankham R (1995) Conservation genetics. Ann. Rev. Genet. 29: 305–327
Gemmill CEC, Ranker TA, Ragone D, Perlman SP, Wood KR (1998) Conservation genetics of the endangered endemic Hawaiian genus Brighamia (Campanulaceae). Amer. J. Bot. 85: 528–539
Gerhardt F, Collinge SK (2003) Exotic plant invasions of vernal pools in the Central Valley of California, USA. J. Biogeo. 30:1043–1052
Godwin ID, Aitken EAB, Smith LW (1997) Application of inter simple sequence repeat (ISSR) markers to plant genetics. Electrophoresis 18: 1524–1528
Gustafson DJ, Gibson DJ, Nickrent DL (2002) Genetic diversity and competitive abilities of Dalea purpurea (Fabaceae) from remnant and restored grasslands. Int. J. of Plant Sci. 163: 979–990
Gustafson DJ, Gibson DJ, Nickrent DL (2004) Conservation genetics of two co-dominant grass species in an endangered grassland ecosystem. J. Appl. Ecol. 41: 389–397
Helenurm K, Parsons LS (1997) Genetic variation and reintroduction of Cordylanthus maritimus ssp. maritimus to Sweetwater Marsh, California. Restor. Ecol. 5: 236–244
Hollingsworth PM, Tebbitt M, Watson KJ, Gornall RJ (1998) Conservation genetics of an arctic species, Saxifraga rivularis L., in Britain. Biol. J. Lin.n Soc. 123: 1–14
Jain A, Apparanda C, Bhalla PL (1999) Evaluation of genetic diversity and genome fingerprinting of Pandorea (Bignoniaceae) by RAPD and inter-SSR PCR. Genome 42: 714–719
Jain SK (1976) Vernal pools: their ecology and conservation. University of California, Davis, CA
Lai J-A, Yang W-C, Hsiao J-Y (2001) An assessment of genetic relationships in cultivated tea clones and native wild tea in Taiwan using RAPD and ISSR markers. Bot. Bull. Acad. Sin. 42: 93–100
Lewontin RC (1972) The apportionment of human diversity. Evol. Biol. 6: 381–398
Li Y-Y, Chen X-Y, Zhang X, Wu T-Y, Lu H-P, Cai Y-W (2005) Genetic differences between wild and artificial populations of Metasequoia glyptostroboides: implications for species recovery. Conserv. Biol. 19: 224–231
Lopez-Pujol J, Bosch M, Simon J, Blanche C (2003) Population genetics and conservation priorities for the critically endangered island endemic Delphinium pentagynum subsp. formenteranum (Ranunculaceae). Biodivers. Conserv. 12: 1937–1951
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol. Ecol. 3: 91–99
Mattner J, Zwako G, Rossetto M, Krauss SL, Dixon KW, Sivasithamparam K (2002) Conservation genetics and implications for restoration of Hemigenia exilis (Lamiaceae), a serpentine endemic from Western Australia. Biol. Conserv. 107: 37–45
McGlaughlin M, Karoly K, Kaye T (2002) Genetic variation and its relationship to population size in reintroduced populations of pink sand verbena, Abronia umbellata subsp. breviflora (Nyctaginaceae). Conserv. Genet. 3: 411–420
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. 70: 3321–3323
Newman D, Pilson D (1997) Increased probability of extinction due to decreased genetic effective populations size: experimental populations of Clarkia pulchella. Evolution 51: 354–362
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol. Ecol. 13: 1143–1155
Nybom H, Bartish IV (2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Persp. Plant Ecol., Evol. Sys. 3: 93–114
Ornduff R (1966) A biosystematic survey of the Goldfield genus␣Lasthenia. In: University of California Publications in Botany
Pavlik BM, Nickrent DL, Howald AM (1993) The recovery of an endangered plant. I. Creating a new population of Amsinckia grandiflora. Conserv. Biol. 7: 510–526
Qian W, Ge S, Hong D-Y (2001) Genetic variation within and among populations of a wild rice Oryza granulata from China detected by RAPD and ISSR markers. Theor. Appl. Genet. 102: 440–449
Ramp JM (2004) Restoration genetics and pollination of the endangered vernal pool endemic Lasthenia conjugens (Asteraceae). University of Colorado, Boulder, USA, PhD thesis
Schneider S, Roessli D, Excoffier L (2000) Arlequin ver. 2.000: A software program for population genetic data analysis. Genetics and Biometry Laboratory, Geneva
Society for Ecological Restoration Working Group (2002) The SER primer on ecological restoration
Stewart CN, Jr., Excoffier L (1996) Assessing population genetic structure and variability with RAPD data: application to Vaccinium macrocarpon (American Cranberry). J. Evol. Biol. 9: 153–171
Stone DR (1990) California’s endemic vernal pool plants: some factors influencing their rarity and endangerment. In: Ikeda DH, Schlising RA (eds) Vernal Pool plants - their habitat and biology. California State University, Studies from the Herbarium No. 8, California State University, Chico, pp. 89–107
Thorp RW (1976) Insect pollination of vernal pool flowers. In: Jain SK (ed) Vernal Pools: Their Ecology and Conservation. Institute of Ecology, University of California, Davis, pp. 36–40
Thorp RW (1990) Vernal pool flowers and host-specific bees. In: Ikeda DH, Schlising RA (eds) Vernal Pool plants - their habitat and biology. California State University, Studies from the Herbarium No. 8, California State University, Chico, pp. 109–122
Travis SE, Proffitt CE, Lowenfeld RC, Mitchell TW (2002) A comparative assessment of genetic diversity among differently-aged populations of Spartina alterniflora on restored versus natural wetlands. Restor. Ecol. 10: 37–42
United States Fish and Wildlife Service (1997) Endangered and threatened wildlife and plants; Endangered status for four plants from vernal pools and mesic areas in Northern California. Federal Register 62: 33029–33038
United States Fish and Wildlife Service (2003) Endangered and threatened wildlife and plants; Final designation of critical habitat for four vernal pool crustaceans and eleven vernal pool plants in California and Southern Oregon. Federal Register 68: 46732–46782
Williams SL, Davis CA (1996) Population genetic analyses of transplanted Eelgrass (Zostera marina) beds reveal reduced genetic diversity in southern California. Restor. Ecol. 4: 163–180
Williams SL, Orth RJ (1998) Genetic diversity and structure of natural and transplanted Eelgrass populations in the Chesapeake and Chincoteague Bays. Estuaries 21: 118–128
Wise CA, Ranker TA, Linhart YB (2002) Modeling problems in conservation genetics with Brassica rapa: Genetic variation and fitness in plants under mild, stable conditions. Conserv. Biol. 16: 1542–1554
Wolfe AD, Liston A (1998) Contributions of PCR-based methods to plant systematics and evolutionary biology. In: Soltis DE, Soltis PS, Doyle JJ, (eds) Molecular Systematics of Plants II DNA Sequencing. Kluwer Academic Pub., Boston, pp. 43–86
Wolfe AD, Randle CP (2001) Relationships within and among species of the holoparasitic genus Hyobanche (Orobanchaceae) inferred from ISSR banding patterns and nucleotide sequences. Sys. Bot. 26: 120–130
Yeh FC, Boyle T, Rongcai Y, Ye Z, Xiyan JM (1999). POPGENE Version 1.31. Edmonton, Alberta
Zedler PH (1990). Life histories of vernal pool vascular plants. In: Ikeda DH, Schlising RA (eds) Vernal pool plants - their habitat and biology. California State University, Studies from the Herbarium No. 8, California State University,Chico, pp. 123–146
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple sequence repeat (SSR)-Anchored polymerase chain reaction amplification. Genomics 20: 176–183
Acknowledgements
We thank Yan Linhart, Andy Martin, and Susan Beatty for valuable comments on the manuscript. This study was funded by grants from the National Science Foundation (DEB-0206088), the Department of Ecology and Evolutionary Biology at the University of Colorado, the University of Colorado Graduate School, the University of Colorado Museum, and the California Native Plant Society. All plant samples were collected under a U.S. Fish and Wildlife permit to SK Collinge (permit TE828382-2). We thank the National Center for Genetic Resources Preservation in Fort Collins, Colorado for allowing J. M. Ramp to use their facilities for DNA extractions. In addition, we thank two undergraduates, Brian Lee and Henry Houghton, who volunteered their time to help with DNA extractions.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Ramp, J.M., Collinge, S.K. & Ranker, T.A. Restoration genetics of the vernal pool endemic Lasthenia conjugens (Asteraceae). Conserv Genet 7, 631–649 (2006). https://doi.org/10.1007/s10592-005-9052-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-005-9052-2