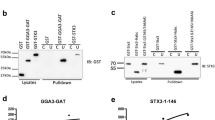
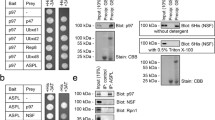
Abstract
Ubiquitination of proteins and their degradation within the proteasome has emerged as the major proteolytic mechanism used by mammalian cells to regulate cytosolic and nuclear protein levels. Substrate ubiquitylation is mediated by ubiquitin (Ub) ligases, also called E3 Ub ligases. HECT-E3 Ub ligases are characterized by the presence of a C-terminal HECT domain that contains the active site for Ub transfer onto substrates. Among the many E3 Ub ligases, the family homologous to E6-Ap C-terminus (HECT) E3 Ub ligases, which includes the yeast protein Rsp5p and the mammalian homolog NEDD4, AIP4/Itch, and Smurf, has been shown to ubiquitylate membrane proteins and, in some instances, to induce their degradation. In this report, we have identified Syntaxin 8 as a binding protein to a novel HECT domain protein, HECT domain containing 3 (HECTd3), by yeast two-hybrid screen. Besides HECT domain, HECTd3 contains an anaphase-promoting complex, subunit 10 (APC10) domain. Our co-immunoprecipitation experiments show that Syntaxin 8 directly interacts with HECTd3 and that the overexpression of HECTd3 promotes the ubiquitination of Syntaxin 8. Immunofluorescence results show that Syntaxin 8 and HECTd3 have similar subcellular localization.




Similar content being viewed by others
References
Ardley HC, Robinson PA (2004) The role of ubiquitin-protein ligases in neurodegenerative disease. Neurodegener Dis 1:71–87. doi:10.1159/000080048
Bogdanovic A, Bennett N, Kieffer S, Louwagie M, Morio T, Garin J, Satre M, Bruckert F (2002) Syntaxin 7, syntaxin 8, Vti1 and VAMP7 (vesicle-associated membrane protein 7) form an active SNARE complex for early macropinocytic compartment fusion in Dictyostelium discoideum. Biochem J 368(Pt 1):29–39
Chin LS, Vavalle JP, Li L (2002) Staring, a novel E3 ubiquitin-protein ligase that targets syntaxin 1 for degradation. J Biol Chem 277:35071–35079. doi:10.1074/jbc.M203300200
Giasson BI, Lee VM (2001) Parkin and the molecular pathways of Parkinson’s disease. Neuron 31:885–888. doi:10.1016/S0896-6273(01)00439-1
Glickman MH, Ciechanover (2002) The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Hicks AA, Petursson H, Jonsson T, Stefansson H, Johannsdottir HS, Sainz J et al (2002) A susceptibility gene for late-onset idiopathic Parkinson’s disease. Ann Neurol 52:549–555. doi:10.1002/ana.10324
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S et al (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392:605–608. doi:10.1038/33416
Kornitzer D, Ciechanover (2000) Modes of regulation of ubiquitin-mediated protein degradation. J Cell Physiol 182:1–11. doi :10.1002/(SICI)1097-4652(200001)182:1<1::AID-JCP1>3.0.CO;2-V
Li YJ, Scott WK, Hedges DJ, Zhang F, Gaskell PC, Nance MA et al (2002) Age at onset in two common neurodegenerative diseases is genetically controlled. Am J Hum Genet 70:985–993. doi:10.1086/339815
Ota T, Suzuki Y, Nishikawa T (2004) Complete sequencing and characterization of 21, 243 full-length human cDNAs. Nat Genet 36:40–45. doi:10.1038/ng1285
Peters SU, Goddard-Finegold J, Beaudet AL, Madduri N, Turcich M, Bacino CA (2004) Cognitive and adaptive behavior profiles of children with Angelman syndrome. Am J Med Genet A 128:110–113. doi:10.1002/ajmg.a.30065
Roessel VP, Elliott DA, Robinson IM, Prokop A, Brand AH (2004) Independent regulation of synaptic size and activity by the anaphase-promoting complex. Cell 119:707–718. doi:10.1016/j.cell.2004.11.028
Schwarz ES, Rosa José L, Scheffner M (1998) Characterization of human hect domain family members and their interaction with UbcH5 and UbcH7. J Biol Chem 273:12148–12154. doi:10.1074/jbc.273.20.12148
Shimura H, Schlossmacher MG, Hattori N, Frosch MP, Trockenbacher A, Schneider R et al (2001) Ubiquitination of a new form of α-synuclein by parkin from human brain: implications for Parkinson’s disease. Science 293:263–269. doi:10.1126/science.1060627
Strausberg RL, Feingold EA, Grouse LH, Derge JG (2002) Generation and initial analysis of more than 15, 000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA 99:16899–16903. doi:10.1073/pnas.242603899
Subramaniam VN, Loh E, Horstmann H, Habermann A, Xu Y, Coe J, Griffiths G, Hong W (2000) Preferential association of syntaxin 8 with the early endosome. J Cell Sci 113(Pt 6):997–1008
Watahiki A, Waki K, Hayatsu N, Shiraki T, Kondo S, Nakamura M et al (2004) Libraries enriched for alternatively spliced exons reveal splicing patterns in melanocytes and melanomas. Nat Methods 1:233–239. doi:10.1038/nmeth719
Yoo MS, Chun HS, Son JJ, DeGiorgio LA, Kim DJ, Peng C et al (2003) Oxidative stress regulated genes in nigral dopaminergic neuronal cells: correlation with the known pathology in Parkinson’s disease. Brain Res Mol Brain Res 110:76–84. doi:10.1016/S0169-328X(02)00586-7
Yu J, Lan J, Zhu Y, Li X, Lai X, Xue Y, Jin C, Huang H (2008) The E3 ubiquitin ligase HECTD3 regulates ubiquitination and degradation of Tara. Biochem Biophys Res Commun 367`(4):805–812
Acknowledgments
We thank Dr. Stephen Matheson of Calvin College for kindly providing the N1E115 neuroblastoma cells. This work was supported by funds from the Van Andel Research Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Kang, L., Bond, W. et al. Interaction Between Syntaxin 8 and HECTd3, a HECT Domain Ligase. Cell Mol Neurobiol 29, 115–121 (2009). https://doi.org/10.1007/s10571-008-9303-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-008-9303-0