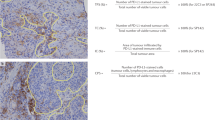
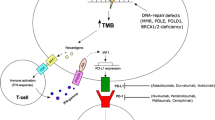
Abstract
With the advent of targeted therapies, there has been a revolution in the treatment of cancer across multiple histologies. Immune checkpoint blockade has made it possible to take advantage of receptor-ligand interactions between immune and tumor cells in a wide spectrum of malignancies. Toxicity in healthy tissue, however, can limit our use of these agents. Immune checkpoint blockade has been approved in advanced melanoma, renal cell cancer, non-small cell lung cancer, relapsed refractory Hodgkin’s lymphoma, and urothelial cancer. Though FDA-approved indications for use of some of these novel agents depend on current protein-based programmed death 1 (PD-1) and programmed death ligand 1 (PD-L1) assays, detection methods come with several caveats. Additional predictive tools must be interrogated to discern responders from non-responders. Some of these include measurement of microsatellite instability, PD-L1 amplification, cluster of differentiation 8 (CD8) infiltrate density, and tumor mutational burden. This review serves to synthesize biomarker detection at the DNA, RNA, and protein level to more accurately forecast benefit from these novel agents.


Similar content being viewed by others
References
Johnson, B.E., Kris, M.G., Berry, L.D., Kwiatkowski, D.J., Lafrate, A.J., Varella-Garcia, M., Wistuba, I.I. (2013). A multicenter effort to identify driver mutations and employ targeted therapy in patients with lung adenocarcinomas: The Lung Cancer Mutation Consortium (LCMC). Journal of Clinical Oncology 2013 ASCO Annual Meeting Abstracts, 13(15).
Patel, S. P., Schwaederle, M., Daniels, G. A., Fanta, P. T., Schwab, R. B., Shimabukuro, K. A., et al. (2015). Molecular inimitability amongst tumors: implications for precision cancer medicine in the age of personalized oncology. Oncotarget, 6(32), 32602–32609. doi:10.18632/oncotarget.5289.
Atkins, M. B., Lotze, M. T., Dutcher, J. P., Fisher, R. I., Weiss, G., Margolin, K., et al. (1999). High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 17(7), 2105–2116.
La-Beck, N. M., Jean, G. W., Huynh, C., Alzghari, S. K., & Lowe, D. B. (2015). Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 35(10), 963–976. doi:10.1002/phar.1643.
Patel, S. P., & Kurzrock, R. (2015). PD-L1 expression as a predictive biomarker in cancer immunotherapy. Molecular Cancer Therapeutics, 14(4), 847–856. doi:10.1158/1535-7163.MCT-14-0983.
Patel, S. P., Osada, T., Osada, K., Hurwitz, H., Lyerly, H. K., & Morse, M. A. (2013). Modulation of immune system inhibitory checkpoints in colorectal cancer. Current Colorectal Cancer Reports, 9(4), 391–397. doi:10.1007/s11888-013-0184-3.
Alexandrov, L. B., Nik-Zainal, S., Wedge, D. C., Aparicio, S. A. J. R., Behjati, S., Biankin, A. V., et al. (2013). Signatures of mutational processes in human cancer. Nature, 500(7463), 415–421. doi:10.1038/nature12477.
Le, D. T., Uram, J. N., Wang, H., Bartlett, B. R., Kemberling, H., Eyring, A. D., et al. (2015). PD-1 blockade in tumors with mismatch-repair deficiency. New England Journal of Medicine, 372(26), 2509–2520. doi:10.1056/NEJMoa1500596.
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer, 12(4), 252–264. doi:10.1038/nrc3239.
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J., & Schreiber, R. D. (2002). Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology, 3(11), 991–998. doi:10.1038/ni1102-991.
Larsen, S. K. (2016). Cellular immune responses towards regulatory cells. Danish Medical Journal, 63(1), B5188.
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine, 366(26), 2443–2454. doi:10.1056/NEJMoa1200690.
Brahmer, J., Reckamp, K. L., Baas, P., Crinò, L., Eberhardt, W. E. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. New England Journal of Medicine, 373(2), 123–135. doi:10.1056/NEJMoa1504627.
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. New England Journal of Medicine, 373(17), 1627–1639. doi:10.1056/NEJMoa1507643.
Herbst, R. S., Baas, P., Kim, D.-W., Felip, E., Pérez-Gracia, J. L., Han, J.-Y., et al. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (London, England), 387(10027), 1540–1550. doi:10.1016/S0140-6736(15)01281-7.
Rosenberg, J. E., Hoffman-Censits, J., Powles, T., van der Heijden, M. S., Balar, A. V., Necchi, A., et al. (2016). Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England), 387(10031), 1909–1920. doi:10.1016/S0140-6736(16)00561-4.
Fehrenbacher, L., Spira, A., Ballinger, M., Kowanetz, M., Vansteenkiste, J., Mazieres, J., et al. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. The Lancet, 387(10030), 1837–1846. doi:10.1016/S0140-6736(16)00587-0.
Massard, C., Gordon, M.S., Sharma, S., Rafil, S., Wainberg, Z.A., Luke, J.J., Curiel, T.J. (2016). Safety and efficacy of durvalumab (MEDI4736), a PD-L1 antibody, in urothelial bladder cancer abstact #4502. Presented at the ASCO 2016, Chicago, Il.
Popat, S., Hubner, R., & Houlston, R. S. (2005). Systematic review of microsatellite instability and colorectal cancer prognosis. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 23(3), 609–618. doi:10.1200/JCO.2005.01.086.
Salipante, S. J., Scroggins, S. M., Hampel, H. L., Turner, E. H., & Pritchard, C. C. (2014). Microsatellite instability detection by next generation sequencing. Clinical Chemistry, 60(9), 1192–1199. doi:10.1373/clinchem.2014.223677.
Lyford-Pike, S., Peng, S., Young, G. D., Taube, J. M., Westra, W. H., Akpeng, B., et al. (2013). Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Research, 73(6), 1733–1741. doi:10.1158/0008-5472.CAN-12-2384.
Schumacher, T. N., & Schreiber, R. D. (2015). Neoantigens in cancer immunotherapy. Science (New York, N.Y.), 348(6230), 69–74. doi:10.1126/science.aaa4971.
Lal, N., Beggs, A. D., Willcox, B. E., & Middleton, G. W. (2015). An immunogenomic stratification of colorectal cancer: implications for development of targeted immunotherapy. Oncoimmunology, 4(3), e976052. doi:10.4161/2162402X.2014.976052.
Palles, C., Cazier, J.-B., Howarth, K. M., Domingo, E., Jones, A. M., Broderick, P., et al. (2012). Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nature Genetics, 45(2), 136–144. doi:10.1038/ng.2503.
van Gool, I. C., Eggink, F. A., Freeman-Mills, L., Stelloo, E., Marchi, E., de Bruyn, M., et al. (2015). POLE proofreading mutations elicit an antitumor immune response in endometrial cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 21(14), 3347–3355. doi:10.1158/1078-0432.CCR-15-0057.
Bosse, Y., Postma, D. S., Sin, D. D., Lamontagne, M., Couture, C., Gaudreault, N., et al. (2012). Molecular signature of smoking in human lung tissues. Cancer Research, 72(15), 3753–3763. doi:10.1158/0008-5472.CAN-12-1160.
Rizvi, N. A., Hellmann, M. D., Snyder, A., Kvistborg, P., Makarov, V., Havel, J. J., et al. (2015). Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (New York, N.Y.), 348(6230), 124–128. doi:10.1126/science.aaa1348.
McGranahan, N., Furness, A. J. S., Rosenthal, R., Ramskov, S., Lyngaa, R., Saini, S. K., et al. (2016). Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (New York, N.Y.), 351(6280), 1463–1469. doi:10.1126/science.aaf1490.
Inoue, Y., Yoshimura, K., Mori, K., Kurabe, N., Kahyo, T., Mori, H., et al. (2016). Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancer. Oncotarget. doi:10.18632/oncotarget.8528.
Ruggiero, E., Nicolay, J. P., Fronza, R., Arens, A., Paruzynski, A., Nowrouzi, A., et al. (2015). High-resolution analysis of the human T-cell receptor repertoire. Nature Communications, 6, 8081. doi:10.1038/ncomms9081.
Tumeh, P. C., Harview, C. L., Yearley, J. H., Shintaku, I. P., Taylor, E. J. M., Robert, L., et al. (2014). PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature, 515(7528), 568–571. doi:10.1038/nature13954.
Groenen, P. J. T. A., Langerak, A. W., van Dongen, J. J. M., & van Krieken, J. H. J. M. (2008). Pitfalls in TCR gene clonality testing: teaching cases. Journal of Hematopathology, 1(2), 97–109. doi:10.1007/s12308-008-0013-9.
Wang, W., Edington, H. D., Rao, U. N. M., Jukic, D. M., Land, S. R., Ferrone, S., & Kirkwood, J. M. (2007). Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 13(5), 1523–1531. doi:10.1158/1078-0432.CCR-06-1387.
Brase, J. C., Wuttig, D., Kuner, R., & Sültmann, H. (2010). Serum microRNAs as non-invasive biomarkers for cancer. Molecular Cancer, 9(1), 306. doi:10.1186/1476-4598-9-306.
Wang, Z., Han, J., Cui, Y., Fan, K., & Zhou, X. (2013). Circulating microRNA-21 as noninvasive predictive biomarker for response in cancer immunotherapy. Medical Hypotheses, 81(1), 41–43. doi:10.1016/j.mehy.2013.03.001.
Ayers, M., Lunceford, J., Nebozhyn, M., Murphy, E., Loboda, A., Albright, A., et al. (2015). Relationship between immune gene signatures and clinical response to PD-1 blockade with pembrolizumab (MK-3475) in patients with advanced solid tumors. Journal for ImmunoTherapy of Cancer, 3(Suppl 2), P80. doi:10.1186/2051-1426-3-S2-P80.
Iglesia, M. D., Parker, J. S., Hoadley, K. A., Serody, J. S., Perou, C. M., & Vincent, B. G. (2016). Genomic analysis of immune cell infiltrates across 11 tumor types. Journal of the National Cancer Institute, 108(11), djw144. doi:10.1093/jnci/djw144.
Partlová, S., Bouček, J., Kloudová, K., Lukešová, E., Zábrodský, M., Grega, M., et al. (2015). Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology, 4(1), e965570. doi:10.4161/21624011.2014.965570.
Wallden, B., Pekker, I., Popa, S., Dowidar, N., Sullivan, A., Hood, T., Danaher, P. (2016). Development and analytical performance of a molecular diagnostic for anti-PD1 response on the nCounterDx analysis system abstract #3034. Presented at the ASCO 2016, Chicago, Il.
Piha-Paul, S., Bennouna, J., Albright, A., Nebozhyn, M., McClanahan, T., Ayers, M., … Ott, P. (2016). T-cell inflamed phenotype gene expression signatures to predict clinical benefit from pembrolizumab across multiple tumor types abstract #1536. Presented at the ASCO 2016, Chicago, Il.
Shen-Orr, S. S., & Gaujoux, R. (2013). Computational deconvolution: extracting cell type-specific information from heterogeneous samples. Current Opinion in Immunology, 25(5), 571–578. doi:10.1016/j.coi.2013.09.015.
Newman, A. M., Liu, C. L., Green, M. R., Gentles, A. J., Feng, W., Xu, Y., et al. (2015). Robust enumeration of cell subsets from tissue expression profiles. Nature Methods, 12(5), 453–457. doi:10.1038/nmeth.3337.
Vinayak, S., Newman, A., Adams, S., Afghahi, A., Jensen, K. C., Badve, S. S., et al. (2015). Abstract P5-04-03: deconvoluting immune cell populations using “in silico flow cytometry” with CIBERSORT: association with neoadjuvant therapy response and genomic instability in TNBC. Cancer Research, 75(9 Supplement), P5–04–03–P5–04–03. doi:10.1158/1538-7445.SABCS14-P5-04-03.
FDA Approves Personalized Lung Cancer Immunotherapy with New Kind of CDx. (2015). Retrieved from https://www.genomeweb.com/molecular-diagnostics/fda-approves-personalized-lung-cancer-immunotherapy-new-kind-cdx.
Borczuk, A. C., & Allen, T. C. (2016). PD-L1 and lung cancer: the era of precision-ish medicine? Archives of Pathology & Laboratory Medicine, 140(4), 351–354. doi:10.5858/arpa.2015-0509-SA.
Schmidt, L. H., Kümmel, A., Görlich, D., Mohr, M., Bröckling, S., Mikesch, J. H., et al. (2015). PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PloS One, 10(8), e0136023. doi:10.1371/journal.pone.0136023.
Bellmunt, J., Mullane, S. A., Werner, L., Fay, A. P., Callea, M., Leow, J. J., et al. (2015). Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Annals of Oncology: Official Journal of the European Society for Medical Oncology/ESMO, 26(4), 812–817. doi:10.1093/annonc/mdv009.
Wu, C., Zhu, Y., Jiang, J., Zhao, J., Zhang, X.-G., & Xu, N. (2006). Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochemica, 108(1), 19–24. doi:10.1016/j.acthis.2006.01.003.
Rizvi, N.A., Chow, L., Borghaei, H., Shen, Y., Harbison, C., Alaparthy, S., Chen, A.C. (2014). Safety and response with nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus erlotinib in patients (pts) with epidermal growth factor receptor mutant (EGFR MT) advanced NSCLC abstract #8022. Presented at the ASCO 2014, Chicago, Il.
Ghebeh, H., Mohammed, S., Al-Omair, A., Qattant, A., Lehe, C., Al-Qudaihi, G., et al. (2006). The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia, 8(3), 190–198. doi:10.1593/neo.05733.
Ritprajak, P., & Azuma, M. (2015). Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncology, 51(3), 221–228. doi:10.1016/j.oraloncology.2014.11.014.
Schmid, P., Hegde, P., Zou, W., Kowanetz, M., Mariathasan, S., Molinero, L., Gadgeel, S. (2016). Association of PD-L2 expression in human tumors with atezolizumab activity abstract #11506. Presented at the ASCO 2016, Chicago, Il.
Woller, N., Gürlevik, E., Fleischmann-Mundt, B., Schumacher, A., Knocke, S., Kloos, A. M., et al. (2015). Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Molecular Therapy: The Journal of the American Society of Gene Therapy, 23(10), 1630–1640. doi:10.1038/mt.2015.115.
Bristol-Meyers Squibb. (n.d.). An open label, randomized phase 3 clinical trial of nivolumab vs therapy of investigator’s choice in recurrent or metastatic platinum-refractory squamous cell carcinoma of the head and neck (SCCHN). (NCT02105636).
Keane, C., Vari, F., Hertzberg, M., Cao, K.-A. L., Green, M. R., Han, E., et al. (2015). Ratios of T-cell immune effectors and checkpoint molecules as prognostic biomarkers in diffuse large B-cell lymphoma: a population-based study. The Lancet Haematology, 2(10), e445–e455. doi:10.1016/S2352-3026(15)00150-7.
Jefferson, E. (2011). FDA Approves new treatment for a type of late-stage skin cancer. US Food and Drug Administration. Retrieved from http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm1193237.htm.
Chambers, C. A., Kuhns, M. S., Egen, J. G., & Allison, J. P. (2001). CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annual Review of Immunology, 19, 565–594. doi:10.1146/annurev.immunol.19.1.565.
Bracarda, S., Altavilla, A., Hamzaj, A., Sisani, M., Marrocolo, F., Del Buono, S., & Danielli, R. (2015). Immunologic checkpoints blockade in renal cell, prostate, and urothelial malignancies. Seminars in Oncology, 42(3), 495–505. doi:10.1053/j.seminoncol.2015.02.004.
Ribas, A., Kefford, R., Marshall, M. A., Punt, C. J. A., Haanen, J. B., Marmol, M., et al. (2013). Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. Journal of Clinical Oncology, 31(5), 616–622. doi:10.1200/JCO.2012.44.6112.
Blank, C., Gajewski, T. F., & Mackensen, A. (2005). Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunology, Immunotherapy: CII, 54(4), 307–314. doi:10.1007/s00262-004-0593-x.
US Food and Drug Administration. (2015). FDA approves Keytruda for advanced non-small cell lung cancer.
Garon, E. B., Rizvi, N. A., Hui, R., Leighl, N., Balmanoukian, A. S., Eder, J. P., et al. (2015). Pembrolizumab for the treatment of non-small-cell lung cancer. New England Journal of Medicine, 372(21), 2018–2028. doi:10.1056/NEJMoa1501824.
US Food and Drug Administration. (2016). Pembrolizumab (KEYTRUDA) checkpoint inhibitor. Retrieved from http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm526430.htm.
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., et al. (2016). Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. New England Journal of Medicine. doi:10.1056/NEJMoa1606774.
US Food and Drug Administration. (2016). Atezolizumab (TECENTRIQ). Retrieved from http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm525780.htm.
Hoffman-La Roche. (2016). A randomized phase 3 study of atezolizumab (an engineered anti-PDL1 antibody) compared to docetaxel in patients with locally advanced or metastatic non-small cell lung cancer who have failed platinum therapy—“OAK.” Retrieved from https://www.clinicaltrials.gov/ct2/show/NCT02008227?term=OAK&rank=2.
Boyerinas, B., Jochems, C., Fantini, M., Heery, C. R., Gulley, J. L., Tsang, K. Y., & Schlom, J. (2015). Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunology Research, 3(10), 1148–1157. doi:10.1158/2326-6066.CIR-15-0059.
Downey, S. G., Klapper, J. A., Smith, F. O., Yang, J. C., Sherry, R. M., Royal, R. E., et al. (2007). Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clinical Cancer Research, 13(22), 6681–6688. doi:10.1158/1078-0432.CCR-07-0187.
Patel, S. P. (2015). Immune checkpoint blockade for lung cancer: state of the art. Translational Cancer Research, 4(4), 415–422.
Wheler, J., Lee, J. J., & Kurzrock, R. (2014). Unique molecular landscapes in cancer: implications for individualized, curated drug combinations. Cancer Research, 74(24), 7181–7184. doi:10.1158/0008-5472.CAN-14-2329.
Wheler, J. J., Parker, B. A., Lee, J. J., Atkins, J. T., Janku, F., Tsimberidou, A. M., et al. (2014). Unique molecular signatures as a hallmark of patients with metastatic breast cancer: implications for current treatment paradigms. Oncotarget, 5(9), 2349–2354. doi:10.18632/oncotarget.1946.
Kurzrock, R., & Giles, F. J. (2015). Precision oncology for patients with advanced cancer: the challenges of malignant snowflakes. Cell Cycle (Georgetown, Tex.), 14(14), 2219–2221. doi:10.1080/15384101.2015.1041695.
Acknowledgments
Yulian Khagi, MD, Razelle Kurzrock, MD, and Sandip Patel, MD, completed the compilation of references, writing, editing, and proofreading of this article. There were no additional parties involved in the completion of this work. This research was funded in part by National Cancer Institute grant P30 CA016672.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Khagi has no conflicts of interest. Dr. Patel receives research funding from MedImmune, Genentech, Pfizer, Amgen, Xcovery, Lilly, and Bristol-Myers Squibb. Dr. Kurzrock receives research funding from Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, and Guardant.
Financial disclosure
Dr. Khagi has no financial disclosures to report. Dr. Patel receives speaking fees from Boehringer Ingelheim and Merck. Dr. Kurzrock receives consultant and advisory board fees from Actuate Therapeutics and Xbiotech. She has an ownership interest in Novena Inc. and Curematch Inc. Dr. Kurzrock is funded in part by the Joan and Irwin Jacobs philanthropic fund.
Rights and permissions
About this article
Cite this article
Khagi, Y., Kurzrock, R. & Patel, S.P. Next generation predictive biomarkers for immune checkpoint inhibition. Cancer Metastasis Rev 36, 179–190 (2017). https://doi.org/10.1007/s10555-016-9652-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-016-9652-y