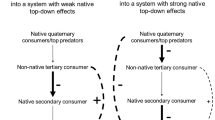
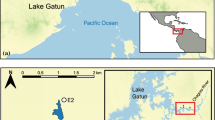
Abstract
Introduced predators have caused some of the largest documented impacts of non-native species. Interactions among predators can have complex effects, leading to both synergistic and antagonistic outcomes. Complex interactions with native predators could play an important role in mediating the impact of non-native predators. We explore the role of the native predator context on the effect of the introduced predatory cladoceran Bythotrephes longimanus. While post-invasion impacts have been well described, studies have largely ignored the role of native predators. We used a field mesocosm experiment to determine whether Bythotrephes’ impact on prey communities is influenced by the presence of the ubiquitous native predatory insect larvae Chaoborus. The two predators exhibited niche complementarity as no change in total zooplankton prey abundance was detected across predator treatments. Rather, copepod abundances increased with decreasing abundances of Chaoborus, while cladocerans decreased with increasing abundances of Bythotrephes. Thus, the replacement of Chaoborus with Bythotrephes led to changes in the overall community structure of the zooplankton prey, but had little effect on prey total abundance. More interestingly, we found evidence of biotic resistance of impact, that is, the impact of Bythotrephes on the cladoceran community was altered when the two predators co-occurred. Specifically, the predation effect of Bythotrephes was more restricted to the shallower regions of the water column in the presence of Chaoborus, leading to a reduced impact on deeper dwelling prey taxa. Overall, our results demonstrate that the native predator context is important when trying to understand the effect of non-native predators and that variation in native predator abundances and assemblages could explain variation in impact across invaded habitats.






Similar content being viewed by others
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anson JR, Dickman CR (2013) Behavioral responses of native prey to disparate predators: naiveté and predator recognition. Oecologia 171:367–377
Audzijonytė A, Väinölä R (2005) Diversity and distributions of circumpolar fresh- and brackish-water Mysis (Crustacea: Mysida): descriptions of M. relicta Lovén, 1862, M. salemaai n. sp., M. segerstralei n. sp. and M. diluviana n. sp., based on molecular and morphological characters. Hydrobiologia 544:89–141
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. doi:10.18637/jss.v067.i01
Beisner BE, Ives AR, Carpenter SR (2003) The effects of an exotic fish invasion on the prey communities of two lakes. J Anim Ecol 72:331–342
Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ (2004) Avian extinction and mammalian introductions on oceanic islands. Science 305:1955–1958
Boudreau SA, Yan ND (2003) The differing crustacean zooplankton communities of Canadian Shield lakes with and without the nonindigenous zooplanktivore Bythotrephes longimanus. Can J Fish Aquat Sci 60:1307–1313
Branstrator DK, Brown ME, Shannon LJ, Thabes M, Heimgartner K (2006) Range expansion of Bythotrephes longimanus in North America: evaluating habitat characteristics in the spread of an exotic zooplankter. Biol Invasions 8:1367–1379
Cairns A, Yan ND, Weisz E, Petruniak J, Hoare J (2007) Operationalizing CAISN Project 1.V Technical Report #2: the large, inland lake, Bythotrephes survey—Limnology, Database Design, and Presence of Bythotrephes in 311 south-central Ontario Lakes. Technical report prepared for the Canadian Aquatic Invasive Species Network
Courchamp F, Chapuis J-L, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Rev 78:347–383
Cox JG, Lima SL (2006) Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680
Dumitru C, Sprules WG, Yan ND (2001) Impact of Bythotrephes longimanus on zooplankton assemblages of Harp Lake, Canada: and assessment based on predator consumption and prey production. Freshw Biol 46:241–251
Dunlop-Hayden KL, Rehage JS (2011) Antipredator behaviour and cue recognition by multiple Everglades prey to a novel cichlid predator. Behaviour 148:795–823
EDDMapS (2016) early detection and distribution mapping system. The University of Georgia—Center for Invasive Species and Ecosystem Health. Available online at http://www.eddmaps.org/. Last accessed 5 Mar 2016
Edgington ES, Onghena P (2007) Randomization tests, 4th edn. Chapman & Hall, London
Elton CS (1958) The ecology of invasions by animals and plants. The University of Chicago Press, Chicago
Foster SE, Sprules WG (2009) Effects of the Bythotrephes invasion on native predatory invertebrates. Limnol Oceanogr 54:757–769
Harrington LA, Harrington AL, Yamaguchi N, Thom MD, Ferreras P, Windham TR, Macdonald DW (2009) The impact of native competitors on an alien invasive: temporal niche shifts to avoid interspecific aggression? Ecology 90:1207–1216
Hothorn T, Bretz F, Westsfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Hulme PE, Pyšek P, Jarošík V, Pergl J, Schaffner U, Vilà M (2013) Bias and error in understanding plan invasion impacts. Trends Ecol Evol (in press)
Ives AR, Cardinale BJ, Snyder WE (2005) A synthesis of subdisciplines: predator-prey interactions, and biodiversity and ecosystem functioning. Ecol Lett 8:102–116
Jokela A (2013) Factors mediating the distribution and impact of the non-native invertebrate predator Bythotrephes longimanus. Dissertation, Queen’s University, Kingston, Ontario, Canada
Jokela A, Arnott SE, Beisner BE (2011) Patterns of Bythotrephes longimanus distribution relative to native macroinvertebrates and zooplankton prey. Biol Invasions 13:2573–2594
Jokela A, Arnott SE, Beisner BE (2013) Influence of light on the foraging impact of an introduced predatory cladoceran, Bythotrephes longimanus. Freshw Biol 58:1946–1957
Kats LB, Ferrer RP (2003) Alien predators and amphibian declines; review of two decades of science and the transition to conservation. Divers Distrib 9:99–110
Kerfoot CW, Yousef F, Hobmeier MM, Maki RP, Jarnagin ST, Churchill JH (2011) Temperature, recreational fishing and diapause egg connections: dispersal of spiny water fleas (Bythotrephes longimanus). Biol Invasions 13:2513–2531
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Lehman JT, Caceres CE (1993) Food-web responses to species invasion by a predatory invertebrate: Bythotrephes in Lake Michigan. Limnol Oceanogr 38:879–891
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, New York
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett 7:975–989
Manly BFJ (2006) Randomization, bootstrap, and Monte Carlo methods in biology, 3rd edn. Chapman & Hall, London
Medina FM, Bonnaud E, Vidal E, Tershy BR, Zavaleta ES, Donlan CJ, Keitt BS, Le Corre M, Horwath SV, Nogales M (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Change Biol 17:3503–3510
Moore MV (1988) Differential use of food resources by the instars of Chaoborus puncipennis. Freshw Biol 19:249–268
Moore MV, Yan ND, Pawson T (1994) Omnivory of the larval phantom midge (Chaoborus spp.) and its potential significance for freshwater planktonic food webs. Can J Zool 72:2055–2065
Muirhead J, Sprules WG (2003) Reaction distance of Bythotrephes longimanus, encounter rate and index of prey risk for Harp Lake, Ontario. Freshw Biol 48:135–146
Nunes AL, Richter-Boix A, Laurila A, Rebelo R (2013) Do anuran larvae respond behaviourally to chemical cues from an invasive crayfish predator? A community-wide study. Oecololgia 171:115–127
Oksanen J, Blanchet FG, Kindt R, Minchin PPR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2012) vegan: community ecology package, R package version 2.0-3. http://CRAN.R-project.org/package=vegan
Pastorok RA (1980) The effects of predator hunger and food abundance on prey selection by Chaoborus larvae. Limnol Oceanogr 25:910–921
R Development Core Team (2012) R: a language and environment for statistical computing, version 2.14.2. R Foundation for Statistical Computing, Vienna
Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL (2013) Progress toward understanding the ecological impacts of non-native species. Ecol Monogr 83:263–282
Rosenheim JA, Wilhoit LR, Armer CA (1993) Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 96:439–449
Salo P, Korpimäki E, Banks PB, Nordström M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc B 274:1237–1243
Schmitz OJ (2007) Predator diversity and trophic interactions. Ecology 88:2415–2426
Sih A, Englund G, Wooster D (1998) Emergent impacts of multiple predators. Trends Ecol Evol 13:350–355
Sih AH, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Soluk DA (1993) Multiple predator effects: predicting combined functional response of stream fish and invertebrate predators. Ecology 74:219–225
Soluk DA, Collins NC (1988) Synergistic interactions between fish and stoneflies: facilitation and interference among stream predators. Oikos 52:94–100
Strecker AL, Arnott SE (2005) Impact of Bythotrephes invasion on zooplankton communities in acid-damaged and recovered lakes on the Boreal Shield. Can J Fish Aquat Sci 62:2450–2462
Strecker AL, Arnott SE (2008) Invasive predator, Bythotrephes, has varied effects on ecosystem function in freshwater lakes. Ecosystems 11:490–503
Strecker AL, Arnott SE, Yan ND, Girard R (2006) Variation in the response of crustacean zooplankton species richness and composition to the invasive predator Bythotrephes longimanus. Can J Fish Aquat Sci 63:2126–2136
Swift MC, Fedorenko AY (1975) Some aspects of prey capture by Chaoborus larvae. Limnol Oceanogr 20:418–425
Valenzuela AEJ, Rey AR, Fasola L, Schiavini A (2013) Understanding the inter-specific dynamics of two co-existing predators in the Tierra del Fuego Archipelago: the native southern river otter and the exotic American mink. Biol Invasions 15:645–656
Vanderploeg HA, Liebig JR, Omair M (1993) Bythotrephes predation on Great Lakes’ zooplankton measured by an in situ method: implications for zooplankton community structure. Archiv Hydrobiol 127:1–8
Wahlström E, Westman E (1999) Planktivory by the predacious cladoceran Bythotrephes longimanus: effects on zooplankton size structure and abundance. Can J Fish Aquat Sci 56:1856–1872
Walsh JR, Carpenter SR, Vander Zanden MJ (2016) Invasive species triggers a massive loss of ecosystem services through a trophic cascade. PNAS 113:4081–4085
Wissel B, Yan ND, Ramcharan CW (2003a) Predation and refugia: implications for Chaoborus abundance and species composition. Freshw Biol 48:1421–1431
Wissel B, Boeing WJ, Ramcharan CW (2003b) Effects of water color on predation regimes and zooplankton assemblages in freshwater lakes. Limnol Oceanogr 48:1965–1976
Yan ND, Leung B, Lewis MA, Peacor SD (2011) The spread, establishment and impacts of the spiny water flea, Bythotrephes longimanus, in temperate North America: a synopsis of the special issue. Biol Invasions 13:2423–2432
Yang D, Gonzalez-Bernal E, Greenlees M, Shine R (2012) Interactions between native and invasive gecko lizards in tropical Australia. Austral Ecol 37:592–599
Young JD, Yan ND (2008) Modification of the diel vertical migration of Bythotrephes longimanus by the cold-water planktivore Coregonus artedi. Freshw Biol 53:981–995
Acknowledgements
Funding for this project was provided by the Canadian Aquatic Invasive Species Network (CAISN), the Department of Fisheries and Oceans Canada, and by an NSERC-CGS scholarship to AJ. Support was also provided through the Queen’s University Summer Work Experience Program. We thank Gary Sprules and Howard Riessen for comments on earlier versions of this manuscript. We thank the staff of the Dorset Environmental Science Centre (DESC) for providing logistical support during our field experiments. We also thank H. Haig, J. Pokorny, S. Feagan, T. Patenaude, D. McGrath, H. and D. Jokela for field assistance, as well as S. MacPhee, H. Haig, C. Symons, N. Talbot, M. Keresztes, and A. Paul for assistance with enumeration of samples.
Authors’ contributions
AJ, SEA and BEB conceived and designed the experiments. AJ performed the experiments, analyzed the data and wrote the manuscript. SEA and BEB provided editorial advice and comments.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1
Linear mixed model results and post hoc tests results examining whether cladoceran taxa abundance varied across the three thermal Strata and if distributions differed between Day and Night. Significant effects (P < 0.05) are highlighted in bold. Analyses were performed on log-transformed data for Daphnia (Control), Bosmina (Control), and Ceriodaphnia (Control, 100% Bythotrephes), on log (x + 1)-transformed data for Daphnia (50% Bythotrephes), Bosmina (50% Bythotrephes, 100% Bythotrephes), Ceriodaphnia (50% Bythotrephes), and Diaphanosoma (all predator treatments), and on square root-transformed data for Daphnia (100% Bythotrephes).
Daphnia
Control | 50% Bythotrephes | 100% Bythotrephes | |||||||
|---|---|---|---|---|---|---|---|---|---|
df | Chi square | P | df | Chi square | P | df | Chi square | P | |
Stratum X Time | 2 | 0.88 | 0.64 | 2 | 6.26 | 0.04 | 2 | 11.97 | 0.003 |
Stratum | 2 | 7.72 | 0.02 | – | – | – | – | – | – |
Time | 1 | 2.16 | 0.14 | – | – | – | – | – | – |
Post Hoc Tests
Control—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | 0.0369 | 0.04 |
Metalimnion | 0.0369 | – | 0.9995 |
Hypolimnion | 0.04 | 0.9995 | – |
50% Bythotrephes—Stratum X Time
Stratum | Day | Night | ||||
|---|---|---|---|---|---|---|
Epilimnion | Metalimnion | Hypolimnion | Epilimnion | Metalimnion | Hypolimnion | |
Day | ||||||
Epilimnion | – | <0.001 | <0.001 | 0.99 | 0.10 | <0.001 |
Metalimnion | <0.001 | – | 1.00 | 0.01 | 1.00 | 0.75 |
Hypolimnion | <0.001 | 1.00 | – | 0.01 | 1.00 | 0.73 |
Night | ||||||
Epilimnion | 0.99 | 0.01 | 0.01 | – | <0.001 | <0.001 |
Metalimnion | 0.10 | 1.00 | 1.00 | <0.001 | – | 0.10 |
Hypolimnion | <0.001 | 0.75 | 0.73 | <0.001 | 0.10 | – |
100% Bythotrephes—Stratum X Time
Stratum | Day | Night | ||||
|---|---|---|---|---|---|---|
Epilimnion | Metalimnion | Hypolimnion | Epilimnion | Metalimnion | Hypolimnion | |
Day | ||||||
Epilimnion | – | 1.00 | 1.00 | 0.54 | 0.91 | <0.001 |
Metalimnion | 1.00 | – | 0.86 | 0.42 | 0.96 | <0.001 |
Hypolimnion | 0.77 | 0.86 | – | 0.03 | 1.00 | 0.03 |
Night | ||||||
Epilimnion | 0.54 | 0.42 | 0.03 | – | 0.07 | <0.001 |
Metalimnion | 0.91 | 0.96 | 1.00 | 0.07 | – | 0.01 |
Hypolimnion | <0.001 | <0.001 | 0.03 | <0.001 | 0.01 | – |
Bosmina
Control | 50% Bythotrephes | 100% Bythotrephes | |||||||
|---|---|---|---|---|---|---|---|---|---|
df | Chi square | P | df | Chi square | P | df | Chi square | P | |
Stratum X Time | 2 | 1.80 | 0.41 | 2 | 2.76 | 0.25 | 2 | 6.24 | 0.04 |
Stratum | 2 | 39.30 | <0.001 | 2 | 13.73 | 0.001 | – | – | – |
Time | 1 | 5.51 | 0.02 | 1 | 1.58 | 0.21 | – | – | – |
Post Hoc Tests
Control—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | <0.001 | <0.001 |
Metalimnion | <0.001 | – | 0.27 |
Hypolimnion | <0.001 | 0.27 | – |
50% Bythotrephes—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | <0.001 | <0.001 |
Metalimnion | <0.001 | – | 0.96 |
Hypolimnion | <0.001 | 0.96 | – |
100% Bythotrephes—Stratum X Time
Stratum | Day | Night | ||||
|---|---|---|---|---|---|---|
Epilimnion | Metalimnion | Hypolimnion | Epilimnion | Metalimnion | Hypolimnion | |
Day | ||||||
Epilimnion | – | 0.82 | 0.97 | 1.00 | 0.85 | 0.06 |
Metalimnion | 0.82 | – | 1.00 | 0.82 | 1.00 | 0.44 |
Hypolimnion | 0.97 | 1.00 | – | 0.95 | 1.00 | 0.24 |
Night | ||||||
Epilimnion | 1.00 | 0.82 | 0.95 | – | 0.43 | 0.001 |
Metalimnion | 0.85 | 1.00 | 1.00 | 0.43 | – | 0.29 |
Hypolimnion | 0.06 | 0.44 | 0.24 | 0.001 | 0.29 | – |
Ceriodaphnia
Control | 50% Bythotrephes | 100% Bythotrephes | |||||||
|---|---|---|---|---|---|---|---|---|---|
df | Chi square | P | df | Chi square | P | df | Chi square | P | |
Stratum X Time | 2 | 0.63 | 0.73 | 2 | 1.35 | 0.51 | 2 | 4.31 | 0.12 |
Stratum | 2 | 41.22 | <0.001 | 2 | 13.85 | <0.001 | 2 | 1.94 | 0.38 |
Time | 1 | 0.91 | 0.34 | 1 | 8.78 | 0.003 | 1 | 0.12 | 0.73 |
Post Hoc Tests
Control—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | 0.495 | <0.001 |
Metalimnion | 0.495 | – | <0.001 |
Hypolimnion | <0.001 | <0.001 | – |
50% Bythotrephes—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | 0.29 | 0.03 |
Metalimnion | 0.29 | – | <0.001 |
Hypolimnion | 0.03 | <0.001 | – |
100% Bythotrephes—NA
Diaphanosoma
Control | 50% Bythotrephes | 100% Bythotrephes | |||||||
|---|---|---|---|---|---|---|---|---|---|
df | Chi square | P | df | Chi square | P | df | Chi square | P | |
Stratum X Time | 2 | 5.43 | 0.07 | 2 | 1.53 | 0.47 | 2 | 0.53 | 0.77 |
Stratum | 2 | 16.88 | <0.001 | 2 | 11.98 | 0.003 | 2 | 2.15 | 0.34 |
Time | 1 | 0.34 | 0.56 | 1 | 0.08 | 0.78 | 1 | 2.06 | 0.15 |
Post Hoc Tests
Control—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | <0.001 | <0.001 |
Metalimnion | <0.001 | – | 0.77 |
Hypolimnion | <0.001 | 0.77 | – |
50% Bythotrephes—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | <0.001 | 0.07 |
Metalimnion | <0.001 | – | 0.18 |
Hypolimnion | 0.07 | 0.18 | – |
100% Bythotrephes—NA
Appendix 2
Linear mixed model results and post hoc tests results examining whether copepods taxa abundance varied across the three thermal Strata and if distributions differed between day and night. Significant effects (P < 0.05) are highlighted in bold. All analyses were performed on log-transformed abundances, except for calanoid copepods in the 50% Bythotrephes treatment, in which no transformation was necessary.
Calanoid
Control | 50% Bythotrephes | 100% Bythotrephes | |||||||
|---|---|---|---|---|---|---|---|---|---|
df | Chi square | P | df | Chi square | P | df | Chi square | P | |
Stratum X Time | 2 | 3.58 | 0.17 | 2 | 7.01 | 0.03 | 2 | 3.83 | 0.15 |
Stratum | 2 | 3.68 | 0.16 | – | – | – | 2 | 4.48 | 0.11 |
Time | 1 | 6.70 | 0.01 | – | – | – | 1 | 2.28 | 0.13 |
Post Hoc Tests
Control—NA
50% Bythotrephes—Stratum X Time
Stratum | Day | Night | ||||
|---|---|---|---|---|---|---|
Epilimnion | Metalimnion | Hypolimnion | Epilimnion | Metalimnion | Hypolimnion | |
Day | ||||||
Epilimnion | – | 0.15 | 1.00 | 0.92 | 1.00 | 0.96 |
Metalimnion | 0.15 | – | 0.27 | 0.76 | 0.28 | 0.67 |
Hypolimnion | 1.00 | 0.27 | – | 0.98 | 1.00 | 1.00 |
Night | ||||||
Epilimnion | 0.92 | 0.76 | 0.98 | – | 0.97 | 1.00 |
Metalimnion | 1.00 | 0.28 | 1.00 | 0.97 | – | 0.99 |
Hypolimnion | 0.96 | 0.67 | 1.00 | 1.00 | 0.99 | – |
100% Bythotrephes—NA
Cyclopoid
Control | 50% Bythotrephes | 100% Bythotrephes | |||||||
|---|---|---|---|---|---|---|---|---|---|
df | Chi square | P | df | Chi square | P | df | Chi square | P | |
Stratum X Time | 2 | 9.67 | 0.01 | 2 | 4.66 | 0.1 | 2 | 7.63 | 0.02 |
Stratum | – | – | – | 2 | 18.12 | <0.001 | – | – | – |
Time | – | – | – | 1 | 4.64 | 0.03 | – | – | – |
Post Hoc Tests
Control—Stratum X Time
Stratum | Day | Night | ||||
|---|---|---|---|---|---|---|
Epilimnion | Metalimnion | Hypolimnion | Epilimnion | Metalimnion | Hypolimnion | |
Day | ||||||
Epilimnion | – | 0.57 | 0.13 | 0.25 | 1.00 | 0.11 |
Metalimnion | 0.57 | – | <0.001 | 0.96 | 0.72 | 0.001 |
Hypolimnion | 0.13 | <0.001 | – | <0.001 | 0.33 | 1.00 |
Night | ||||||
Epilimnion | 0.25 | 0.96 | <0.001 | – | 0.08 | <0.001 |
Metalimnion | 1.00 | 0.72 | 0.33 | 0.08 | – | 0.03 |
Hypolimnion | 0.11 | 0.001 | 1.00 | <0.001 | 0.03 | – |
50% Bythotrephes—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | 0.81 | <0.001 |
Metalimnion | 0.81 | – | <0.001 |
Hypolimnion | <0.001 | <0.001 | – |
100% Bythotrephes—Stratum X Time
Stratum | Day | Night | ||||
|---|---|---|---|---|---|---|
Epilimnion | Metalimnion | Hypolimnion | Epilimnion | Metalimnion | Hypolimnion | |
Day | ||||||
Epilimnion | – | 0.32 | <0.001 | 0.97 | 0.98 | <0.001 |
Metalimnion | 0.32 | – | <0.001 | 0.79 | 0.07 | <0.001 |
Hypolimnion | <0.001 | <0.001 | – | <0.001 | 0.01 | 1.00 |
Night | ||||||
Epilimnion | 0.97 | 0.79 | <0.001 | – | 0.70 | <0.001 |
Metalimnion | 0.98 | 0.07 | 0.01 | 0.70 | – | 0.003 |
Hypolimnion | <0.001 | <0.001 | 1.00 | <0.001 | 0.003 | – |
Appendix 3
Linear mixed model results and post hoc tests results examining whether Chaoborus abundance varied across the three thermal Strata and if distributions differed between day and night. Significant effects (P < 0.05) are highlighted in bold. Analyses were performed on square root-transformed data for the control enclosures and on log-transformed data for the 50% Bythotrephes enclosures.
Chaoborus
Control | 50% Bythotrephes | |||||
|---|---|---|---|---|---|---|
df | Chi square | P | df | Chi square | P | |
Stratum X Time | 2 | 1.08 | 0.58 | 1 | 0.74 | 0.39 |
Stratum | 2 | 17.41 | <0.001 | 1 | 10.00 | 0.002 |
Time | 1 | 0.68 | 0.41 | 1 | 0.07 | 0.79 |
Post Hoc Tests
Control—Stratum
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | – | 0.91 | <0.001 |
Metalimnion | 0.91 | – | <0.001 |
Hypolimnion | <0.001 | <0.001 | – |
50% Bythotrephes—Stratum X Time
Stratum | Epilimnion | Metalimnion | Hypolimnion |
|---|---|---|---|
Epilimnion | NA | NA | NA |
Metalimnion | NA | – | <0.001 |
Hypolimnion | NA | <0.001 | – |
Rights and permissions
About this article
Cite this article
Jokela, A., Arnott, S.E. & Beisner, B.E. Biotic resistance of impact: a native predator (Chaoborus) influences the impact of an invasive predator (Bythotrephes) in temperate lakes. Biol Invasions 19, 1495–1515 (2017). https://doi.org/10.1007/s10530-017-1374-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1374-8