Abstract
An acetate-negative mutant of Yarrowia lipolytica Wratislavia K1 was selected that, when grown with 300 g raw glycerol l−1 at pH 3, produced 170 g erythritol l−1 after 7 days, corresponding to a 56% yield and a productivity of 1 g l−1 h−1. The Wratislavia K1 strain did not produce citric acid.
Similar content being viewed by others
Introduction
Erythritol is a biological sweetener with applications in food and pharmaceuticals. Not only animal toxicological but also clinical studies have consistently demonstrated its safety even when consumed on a daily basis in high amounts (Kawanabe et al. 1992). Large-scale production of erythritol uses fermentative processes with pure glucose, sucrose and dextrose from chemically and enzymatically hydrolyzed wheat and corn starches used as major carbon sources (Aoki et al. 1993; Marina et al. 1993). Erythritol is produced by fermentation involving yeast-like fungi such as Trigonopsis variabilis (Kim et al. 1997), Trichosporon sp. (Park et al. 1998), Torula sp. (Kim et al. 2000), Candida magnoliae (Ryu et al. 2000) and Moniliella sp. (Lin et al. 2001). Leuconostoc oenos can also produce erythritol but only under anaerobic conditions (Veigada-Cunha et al. 1992). A high initial concentration of glucose favors erythritol production by osmophilic microorganisms. Generally, an increase in the initial glucose concentration increases the production rate and yield in a batch process if the microorganisms can tolerate a higher concentration of sugar and a higher osmotic pressure. Erythritol has been produced commercially using a mutant of Aureobasidium (Ishizuka et al. 1989). The mutant produced erythritol at 1.8 g l−1 h−1 with a 44% yield in a medium containing 40% (w/v) glucose. Although erythritol fermentation in synthetic media containing glucose or fructose has extensively been studied, little information is available about the production of this compound by Yarrowia lipolytica from glycerol. New uses of glycerol and new microbial transformations to interesting products are of increasing importance (Papanikolaou et al. 2008; Koutinas et al. 2007). What seems to be important for an economically competitive fermentation process is the ability of Y. lipolytica to grow and to produce erythritol on renewable products from industrial processes (such as raw glycerol discharged after biodiesel manufacture), which are low-cost carbon substrates. A cost reduction in erythritol production can be achieved by using less expensive substrates. We have recently reported that an acetate-negative mutant of Y. lipolytica Wratislavia K1 has the ability of simultaneously producing high amounts of erythritol and citric acid in media containing glycerol, at pH 5.5 which is optimal for citric acid biosynthesis by Y. lipolytica (Rymowicz et al. 2006, 2008). In the current study, we evaluate the dynamics and yield of erythritol production from raw glycerol by the strain Wratislavia K1 at various pH, and thereafter investigate, in fed-batch experiments, the effect of the optimal pH on the production of erythritol by other strains belonging to the species Y. lipolytica.
Materials and methods
Microorganisms and media
The following strains were used in this study: three acetate negative mutants of Yarrowia lipolytica: Wratislavia AWG7, Wratislavia 1.31 and Wratislavia K1, one mutant of Y. lipolytica 8661 UV1, and two wild strains of Y. lipolytica: A-101 and 1.22. All were from Wroclaw University of Environmental and Life Sciences, Poland. The growth medium for inoculum preparation contained: 50 g glycerol l−1, 3 g yeast extract l−1, 3 g malt extract l−1 and 5 g Bactopepton l−1. Erythritol production was conducted in a medium consisting of 150 g glycerol l−1, 3 g NH4Cl l−1, 1 g MgSO4·7H2O l−1, 0.2 g KH2PO4 l−1, and 1 g yeast extract l−1. After 48 h of cultivation, raw glycerol solution (86% w/v) was added (at a constant feeding rate of 1.4 g h−1) until a total of 300 g glycerol l−1 was obtained.
Culture conditions
A seed culture was grown in a 300 ml flask (containing 50 ml of growth medium) on a shaker at 30°C for 3 days. An inoculum of 200 ml was introduced into the fermenter, which contained 1.8 l of the production medium. All fed-batch cultures were performed in a 5 l jar fermenter (Biostat B Plus, Sartorius, Germany) with a working volume of 2 l at 30°C for 7 days. The aeration rate was 0.6 l min−1. The stirrer speed was adjusted to 800 rpm and the pH was maintained automatically at 3 by the addition of NaOH (20% w/v). All the cultures were conducted in two replications.
Analytical methods
The biomass was determined gravimetrically after drying in a drier at 105°C. The concentrations of erythritol, mannitol, glycerol and citric acid were measured by HPLC using an Aminex HPX87H Organic Acid column coupled to a UV detector at 210 nm and an CORONE detector. The column was eluted at 65°C with 15 mM of trifluoroacetic acid at 0.6 ml min−1.
Results and discussion
Effect of the medium pH on the production of erythritol
Our recent investigations have shown that an acetate-negative mutant of Y. lipolytica Wratislavia K1, can produce simultaneously high amounts of erythritol and citric acid under nitrogen-limited conditions, with glycerol as the carbon source, at the pH of 5.5, which is optimal for citrate biosynthesis (Rymowicz et al. 2008). The effect of the pH of the medium on the production of erythritol by this strain in a glycerol containing medium (total concentration 300 g l−1) was examined using media of a pH of 2.5–6.5. As shown in Fig. 1, the increase in the pH of the fermentation medium from 2.5 to 6.5 resulted in a significant decrease in erythritol concentration and an increase in the content of citric acid (an unwanted product in this process) up to 106–108 g l−1 at the pH of 5–5.5. Fed-batch cultivation of Wratislavia K1 at pH 3 gave the highest erythritol production at 170 g l−1, a value comparable with those reported for batch and fed-batch fermentations by various yeasts using glucose as the sole substrate (Aoki et al. 1993; Kim et al. 2000; Lee et al. 2000). The strain used in the present study produced no citric acid at pH values between 2.5 and 3. Moreover, at such low pH, Wratislavia K1 strain stimulated the production of mannitol (12 to 13.5 g l−1). A 56–70% decrease in the concentration of mannitol was observed in the fed-batch cultures from pH 5 to 6.5. Yang et al. (1999) have reported that C. magnoliae isolated from honey combs produced erythritol in the glucose containing medium without mannitol formation. With other yeasts, e.g. Trichosporon sp. and Aureobasidium sp., as shown by Park et al. (1998) and Ishizuka et al. (1989), also polyols such as ribitol, glycerol and/or ethanol were produced, which leads to a decrease in the erythritol conversion yield. In addition, the authors mentioned have observed that the initial pH of the fermentation medium for the high yield production of erythritol was set at higher values, which ranged from 5.5 to 7. This pH range also stimulated the production of mannitol by various yeasts (Baek et al. 2003).
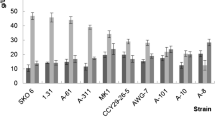
Comparison of erythritol production by various strains of Y. lipolytica
The fermentation characteristics of the various strains of Yarrowia that were grown in fed-batch cultures at pH 3 are summarized in Table 1. The key fermentation parameters, the final concentration of erythritol, volumetric erythritol production rate, as well as erythritol yields during several fed-batch fermentation processes depended on the yeast strains used and ranged from 93.5 to 170 g l−1, 0.51 to 1 g l−1 h−1 and 0.31 to 0.56 g g−1, respectively. Of all the erythritol-synthesizing strains examined, only Wratislavia K1 failed to produce citric acid under these conditions. The other strains synthesized citric acid from 14 g l−1 (8661 UV1 strain) to 54 g l−1 (1.22 strain) and higher concentrations of mannitol, varying from 19 g l−1 (Wratislavia AWG7) to 40.5 g l−1 (8661 UV1 strain).
To investigate the ability of the Wratislavia K1 strain to produce erythritol, glucose which is a major substrate for erythritol biosynthesis by other yeasts (Yang et al. 1999; Ishizuka et al. 1989), was used as a carbon source. The fermentation characteristics of this strain, with the same amount of glucose and glycerol in the production medium (300 g l−1) are depicted in Fig. 2. The patterns of cell growth and erythritol production were almost the same for both substrates. Erythritol was produced simultaneously with cell growth, suggesting that erythritol production is growth-relatated. The most efficient substrate for erythritol production by the Wratislavia K1 strain was glycerol. Glucose can also be converted into erythritol but with lower conversion yield. At the end of fermentation with glucose (190 h), erythritol was at 23 g l−1, whereas erythritol productivity and yield amounted to 0.09 g l−1 h−1 and 0.14 g g−1, respectively. This corresponded to a 7.1- and 6.2-fold decrease in productivity and yield, respectively, compared with the fed-batch culture on glycerol. In addition, Wratislavia K1 produced a higher amount of mannitol (23 g l−1) in a glucose medium.
Time profiles of biomass (+), erythritol (●), mannitol (■), glycerol (○) and glucose (□) for the Y. lipolytica strain Wratislavia K1 during fed-batch cultures on glycerol (a) and glucose (b) at pH 3.0. Glycerol or glucose solution was continuously fed into the fermenter until the total concentration of 300 g l−1 was reached after the initiation of the fed-batch mode indicated by an arrow. Data from duplicate fermentations are shown
The results presented in this paper clearly indicate that the production of erythritol from raw glycerol by the newly screened strain of Y. lipolytica Wratislavia K1 is, in several respects, superior to erythritol production by other strains (Ishizuka et al. 1989; Park et al. 1998; Lin et al. 2001). Firstly, we obtained a high erythritol concentration (170 g l−1) and yield (0.56 g g−1) at pH 3. Secondly, under these conditions, our strain only synthesized mannitol in small quantities and did not produce citric acid (both compounds being by-products in this process). These findings make a valuable contribution to the development of a cost-effective fermentation based on renewable resources.
References
Aoki MAY, Pastore GM, Park YK (1993) Microbial transformation of sucrose and glucose to erythritol. Biotechnol Lett 15:338–383
Baek H, Song KH, Park SM, Kim SY, Hyun HH (2003) Role of glucose in the bioconversion of fructose into mannitol by Candida magnoliae. Biotechnol Lett 25:761–765
Ishizuka H, Wako K, Kasumi T, Sasaki T (1989) Breeding of a mutant of Aureobasidium sp with high erythritol production. J Ferment Bioeng 68:310–314
Kawanabe J, Hiraswa M, Takeuchi T, Oda T, Ikeda T (1992) Non-cariogenicity of erythritol as a substrate. Caries Res 26:358–362
Kim SY, Lee KH, Kim JH, Oh DK (1997) Erythritol production by controlling osmotic pressure in Trigonopsis variabilis. Biotechnol Lett 19(8):727–729
Kim KA, Noh BS, Lee JK, Kim SY, Park YC, Oh DK (2000) Optimization of culture conditions for erythritol production by Torula sp. J Microbiol Biotechnol 10:69–74
Koutinas AA, Wang RH, Webb C (2007) The biochemurgist–Bioconversion of agricultural raw materials for chemical production. Biofuels Bioprod Bioref 1(1):24–38
Lee JK, Ha SJ, Kim SY, Oh DK (2000) Increased erythritol production in Torula sp by Mn2+ and Cu2+. Biotechnol Lett 22:983–986
Lin SJ, Wen CY, Liau JC, Chu WS (2001) Screening and production of erythritol by newly isolated osmophilic yeast-like fungi. Process Biochem 36:1249–1258
Marina AYA, Glaucia MP, Park YK (1993) Microbial transformation of sucrose and glucose to erythritol. Biotechnol Lett 15:383–388
Papanikolaou S, Fakas S, Fick M, Chevalot I, Galiotou-Panayotou M (2008) Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1, 3-propanediol, citric acid and single cell oil. Biomass Bioeng 32:60–71
Park J, Seo B, Kim J, Park J (1998) Production of erythritol in fed-batch cultures of Trichosporon sp. J Ferment Bioeng 86(6):577–580
Rymowicz W, Rywińska A, Żarowska B, Juszczyk P (2006) Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica. Chem Pap 60(5):391–394
Rymowicz W, Rywińska A, Gładkowski W (2008) Simultaneous production of citric acid and erythritol from crude glycerol by Yarrowia lipolytica Wratislavia K1. Chem Pap 62(3):239–246
Ryu YW, Park CY, Park JB, Kim SY, Seo JH (2000) Optimization of erythritol production by Candida magnoliae in fed-batch culture. J Ind Microbiol Biotechnol 25:100–103
Veigada-Cunha M, Firme P, San-Romao MV, Santos H (1992) Application of 13C nuclear magnetic resonance to elucidate the unexpected biosynthesis of erythritol by Leuconostoc oenos. Appl Environ Microbiol 58:2271–2279
Yang SW, Park JB, Han NS, Ryu YW, Seo JH (1999) Production of erythrytol from glucose by an osmophilic mutant of Candida magnoliae. Biotechnol Lett 21:887–890
Acknowledgment
This work was financed by the Ministry of Sciences and Higher Education of Poland under Project No. 2P06T 044 30.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rymowicz, W., Rywińska, A. & Marcinkiewicz, M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica . Biotechnol Lett 31, 377–380 (2009). https://doi.org/10.1007/s10529-008-9884-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9884-1