Abstract
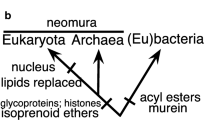
The PVC superphylum is a grouping of distinct phyla of the domain bacteria proposed initially on the basis of 16S rRNA gene sequence analysis. It consists of a core of phyla Planctomycetes, Verrucomicrobia and Chlamydiae, but several other phyla have been considered to be members, including phylum Lentisphaerae and several other phyla consisting only of yet-to-be cultured members. The genomics-based links between Planctomycetes, Verrucomicrobia and Chlamydiae have been recently strengthened, but there appear to be other features which may confirm the relationship at least of Planctomycetes, Verrucomicrobia and Lentisphaerae. Remarkably these include the unique planctomycetal compartmentalized cell plan differing from the cell organization typical for bacteria. Such a shared cell plan suggests that the common ancestor of the PVC superphylum members may also have been compartmentalized, suggesting this is an evolutionarily homologous feature at least within the superphylum. Both the PVC endomembranes and the eukaryote-homologous membrane-coating MC proteins linked to endocytosis ability in Gemmata obscuriglobus and shared by PVC members suggest such homology may extend beyond the bacteria to the Eukarya. If so, either our definition of bacteria may have to change or PVC members admitted to be exceptions. The cases for and against considering the PVC superphylum members as exceptions to the bacteria are discussed, and arguments for them as exceptions presented. Recent critical analysis has favoured convergence and analogy for explaining eukaryote-like features in planctomycetes and other PVC organisms. The case is made for constructing hypotheses leaving the possibility of homology and evolutionary links to eukaryote features open. As the case of discovery of endocytosis-like protein uptake in planctomycetes has suggested, this may prove a strong basis for the immediate future of experimental research programs in the PVC scientific community.





Similar content being viewed by others
References
Albrecht W, Fischer A et al (1987) Verrucomicrobium spinosum, an eubacterium representing an ancient line of descent. Syst Appl Microbiol 10:57–62
Belzer C, de Vos WM (2012) Microbes inside—from diversity to function: the case of Akkermansia. ISME J 6(8):1449–1458
Bode HB, Zeggel B et al (2003) Steroid biosynthesis in prokaryotes: identification of myxobacterial steroids and cloning of the first bacterial 2,3(S)-oxidosqualene cyclase from the myxobacterium Stigmatella aurantiaca. Mol Microbiol 47(2):471–481
Chistoserdova L, Jenkins C et al (2004) The enigmatic planctomycetes may hold a key to the origins of methanogenesis and methylotrophy. Mol Biol Evol 21(7):1234–1241
Cho JC, Vergin KL et al (2004) Lentisphaera araneosa gen. nov., sp. nov, a transparent exopolymer producing marine bacterium, and the description of a novel bacterial phylum, Lentisphaerae. Environ Microbiol 6(6):611–621
Choi A, Yang SJ et al (2013) Lentisphaera marina sp. nov., and emended description of the genus Lentisphaera. Int J Syst Evol Microbiol 63(Pt 4):1540–1544
Choo YJ, Lee K et al (2007) Puniceicoccus vermicola gen. nov., sp. nov., a novel marine bacterium, and description of Puniceicoccaceae fam. nov., Puniceicoccales ord. nov., Opitutaceae fam. nov., Opitutales ord. nov. and Opitutae classis nov. in the phylum Verrucomicrobia. Int J Syst Evol Microbiol 57(Pt 3):532–537
de Duve C (2007) The origin of eukaryotes: a reappraisal. Nat Rev Genet 8(5):395–403
DeGrasse JA, DuBois KN et al (2009) Evidence for a shared nuclear pore complex architecture that is conserved from the last common eukaryotic ancestor. Mol Cell Proteomics 8(9):2119–2130
Devos DP (2012) Regarding the presence of membrane coat proteins in bacteria: confusion? What confusion? BioEssays 34(1):38–39
Devos D, Dokudovskaya S et al (2004) Components of coated vesicles and nuclear pore complexes share a common molecular architecture. PLoS Biol 2(12):e380
Dunfield PF, Yuryev A et al (2007) Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 450(7171):879–882
Dunfield PF, Tamas I et al (2012) Electing a candidate: a speculative history of the bacterial phylum OP10. Environ Microbiol 14(12):3069–3080
Everard A, Belzer C et al (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110(22):9066–9071
Fieseler L, Horn M et al (2004) Discovery of the novel candidate phylum Poribacteria in marine sponges. Appl Environ Microbiol 70(6):3724–3732
Fuerst JA (2005) Intracellular compartmentation in planctomycetes. Annu Rev Microbiol 59:299–328
Fuerst JA, Sagulenko E (2010) Protein uptake by bacteria: an endocytosis-like process in the planctomycete Gemmata obscuriglobus. Commun Integr Biol 3(6):572–575
Fuerst JA, Sagulenko E (2011) Beyond the bacterium: planctomycetes challenge our concepts of microbial structure and function. Nat Rev Microbiol 9(6):403–413
Fuerst JA, Sagulenko E (2012) Keys to eukaryality: planctomycetes and ancestral evolution of cellular complexity. Front Microbiol 3:167
Fuerst JA, Sagulenko E (2013) Nested bacterial boxes: nuclear and other intracellular compartments in planctomycetes. J Mol Microbiol Biotechnol 23(1–2):95–103
Fuerst JA, Webb RI (1991) Membrane-bounded nucleoid in the eubacterium Gemmata obscuriglobus. Proc Natl Acad Sci USA 88(18):8184–8188
Glockner FO, Kube M et al (2003) Complete genome sequence of the marine planctomycete Pirellula sp. strain 1. Proc Natl Acad Sci USA 100(14):8298–8303
Glockner J, Kube M et al (2010) Phylogenetic diversity and metagenomics of candidate division OP3. Environ Microbiol 12(5):1218–1229
Greub G, Raoult D (2002) Crescent bodies of Parachlamydia acanthamoeba and its life cycle within Acanthamoeba polyphaga: an electron micrograph study. Appl Environ Microbiol 68(6):3076–3084
Griffiths E, Gupta RS (2007) Phylogeny and shared conserved inserts in proteins provide evidence that Verrucomicrobia are the closest known free-living relatives of chlamydiae. Microbiology 153(Pt 8):2648–2654
Gupta RS (2008) Complete genome sequence of the extremely acidophilic methanotroph isolate V4, Methylacidiphilum infernorum, a representative of the bacterial phylum Verrucomicrobia. Biol Direct 3:26
Gupta RS, Bhandari V et al (2012) Molecular signatures for the PVC clade (Planctomycetes, Verrucomicrobia, Chlamydiae, and Lentisphaerae) of bacteria provide insights into their evolutionary relationships. Front Microbiol 3:327
Hedlund BP, Gosink JJ et al (1997) Verrucomicrobia div. nov., a new division of the bacteria containing three new species of Prosthecobacter. Antonie Van Leeuwenhoek 72(1):29–38
Hou S, Makarova KS et al (2008) Complete genome sequence of the extremely acidophilic methanotroph isolate V4, Methylacidiphilum infernorum, a representative of the bacterial phylum Verrucomicrobia. Biol Direct 3:26
Hugenholtz P, Goebel BM et al (1998a) Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity. J Bacteriol 180(18):4765–4774
Hugenholtz P, Pitulle C et al (1998b) Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol 180(2):366–376
Jenkins C, Kedar V et al (2002) Gene discovery within the planctomycete division of the domain bacteria using sequence tags from genomic DNA libraries. Genome Biol 3(6): RESEARCH0031
Jenkins C, Samudrala R et al (2002) Genes for the cytoskeletal protein tubulin in the bacterial genus Prosthecobacter. Proc Natl Acad Sci USA 99(26):17049–17054
Jogler C, Glockner FO et al (2011) Characterization of Planctomyces limnophilus and development of genetic tools for its manipulation establish it as a model species for the phylum Planctomycetes. Appl Environ Microbiol 77(16):5826–5829
Jogler C, Waldmann J et al (2012) Identification of proteins likely to be involved in morphogenesis, cell division, and signal transduction in Planctomycetes by comparative genomics. J Bacteriol 194(23):6419–6430
Kamneva OK, Liberles DA et al (2010) Genome-wide influence of indel substitutions on evolution of bacteria of the PVC superphylum, revealed using a novel computational method. Genome Biol Evol 2:870–886
Kamneva OK, Knight SJ et al (2012) Analysis of genome content evolution in PVC bacterial super-phylum: assessment of candidate genes associated with cellular organization and lifestyle. Genome Biol Evol 4(12):1375–1390
Khadem AF, Pol A et al (2010) Nitrogen fixation by the verrucomicrobial methanotroph Methylacidiphilum fumariolicum SolV. Microbiology 156(Pt 4):1052–1059
König E, Schlesner H et al (1984) Cell-wall studies on budding bacteria of the Planctomyces/Pasteuria group and on a Prosthecomicrobium sp. Arch Microbiol 138(3):200–205
Kostanjsek R, Strus J et al (2004) Candidatus Rhabdochlamydia porcellionis, an intracellular bacterium from the hepatopancreas of the terrestrial isopod Porcellio scaber (Crustacea: Isopoda). Int J Syst Evol Microbiol 54(Pt 2):543–549
Kulichevskaya IS, Baulina OI et al (2009) Zavarzinella formosa gen. nov., sp. nov., a novel stalked, Gemmata-like planctomycete from a Siberian peat bog. Int J Syst Evol Microbiol 59(Pt 2):357–364
Lee KC, Webb RI et al (2009) Phylum Verrucomicrobia representatives share a compartmentalized cell plan with members of bacterial phylum Planctomycetes. BMC Microbiol 9:5
Lienard J, Croxatto A et al (2011) Estrella lausannensis, a new star in the Chlamydiales order. Microbes Infect 13(14–15):1232–1241
Liesack W, Stackebrandt E (1989) Evidence for unlinked rrn operons in the planctomycete Pirellula marina. J Bacteriol 171(9):5025–5030
Liesack W, Konig H et al (1986) Chemical composition of the peptidoglycan-free cell envelopes of budding bacteria of the Pirella/Planctomyces group. Arch Microbiol 145(4):361–366
Limam RD, Bouchez T et al (2010) Detection of WWE2-related Lentisphaerae by 16S rRNA gene sequencing and fluorescence in situ hybridization in landfill leachate. Can J Microbiol 56(10):846–852
Lindsay MR, Webb RI et al (1995) Effects of fixative and buffer on morphology and ultrastructure of a fresh-water planctomycete, Gemmata obscuriglobus. J Microbiol Methods 21(1):45–54
Lindsay MR, Webb RI et al (1997) Pirellulosomes: a new type of membrane-bounded cell compartment in planctomycete bacteria of the genus Pirellula. Microbiology 143:739–748
Lindsay MR, Webb RI et al (2001) Cell compartmentalisation in planctomycetes: novel types of structural organisation for the bacterial cell. Arch Microbiol 175(6):413–429
Lonhienne TG, Sagulenko E et al (2010) Endocytosis-like protein uptake in the bacterium Gemmata obscuriglobus. Proc Natl Acad Sci USA 107(29):12883–12888
Martin-Galiano AJ, Oliva MA et al (2011) Bacterial tubulin distinct loop sequences and primitive assembly properties support its origin from a eukaryotic tubulin ancestor. J Biol Chem 286(22):19789–19803
McCoy AJ, Adams NE et al (2006) l, l-diaminopimelate aminotransferase, a trans-kingdom enzyme shared by Chlamydia and plants for synthesis of diaminopimelate/lysine. Proc Natl Acad Sci USA 103(47):17909–17914
McInerney JO, Martin WF et al (2011) Planctomycetes and eukaryotes: a case of analogy not homology. BioEssays 33(11):810–817
Ouellette SP, Karimova G et al (2012) Chlamydia co-opts the rod shape-determining proteins MreB and Pbp2 for cell division. Mol Microbiol 85(1):164–178
Palleja A, Garcia-Vallve S et al (2009) Adaptation of the short intergenic spacers between co-directional genes to the Shine-Dalgarno motif among prokaryote genomes. BMC Genomics 10:537
Patt TE, Hanson RS (1978) Intracytoplasmic membrane, phospholipid, and sterol content of Methylobacterium organophilum cells grown under different conditions. J Bacteriol 134(2):636–644
Pearson A, Budin M et al (2003) Phylogenetic and biochemical evidence for sterol synthesis in the bacterium Gemmata obscuriglobus. Proc Natl Acad Sci USA 100(26):15352–15357
Pilhofer M, Rosati G et al (2007) Coexistence of tubulins and ftsZ in different Prosthecobacter species. Mol Biol Evol 24(7):1439–1442
Pilhofer M, Rappl K et al (2008) Characterization and evolution of cell division and cell wall synthesis genes in the bacterial phyla Verrucomicrobia, Lentisphaerae, Chlamydiae, and Planctomycetes and phylogenetic comparison with rRNA genes. J Bacteriol 190(9):3192–3202
Pilhofer M, Ladinsky MS et al (2011) Microtubules in bacteria: ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton. PLoS Biol 9(12):e1001213
Pol A, Heijmans K et al (2007) Methanotrophy below pH 1 by a new Verrucomicrobia species. Nature 450(7171):874–878
Qiu YL, Muramatsu M et al (2013) Oligosphaera ethanolica gen. nov., sp. nov., an anaerobic, carbohydrate-fermenting bacterium isolated from methanogenic sludge, and description of Oligosphaeria classis nov. in the phylum Lentisphaerae. Int J Syst Evol Microbiol 63(Pt 2):533–539
Rinke C, Schwientek P et al (2013) Insights into the phylogeny and coding potential of microbial dark matter. Nature 499(7459):431–437
Santarella-Mellwig R, Franke J et al (2010) The compartmentalized bacteria of the planctomycetes-verrucomicrobia-chlamydiae superphylum have membrane coat-like proteins. PLoS Biol 8(1):e1000281
Santarella-Mellwig R, Pruggnaller S et al (2013) Three-dimensional reconstruction of bacteria with a complex endomembrane system. PLoS Biol 11(5):e1001565
Schlesner H (1987) Verrucomicrobium spinosum gen. nov., sp.nov., a fimbriated prosthecate bacterium. Syst Appl Microbiol 10:54–56
Schlieper D, Oliva MA et al (2005) Structure of bacterial tubulin BtubA/B: evidence for horizontal gene transfer. Proc Natl Acad Sci USA 102(26):9170–9175
Schouten S, Bowman JP et al (2000) Sterols in a psychrophilic methanotroph, Methylosphaera hansonii. FEMS Microbiol Lett 186(2):193–195
Sinninghe Damste JS, Rijpstra WI et al (2005) Structural identification of ladderane and other membrane lipids of planctomycetes capable of anaerobic ammonium oxidation (anammox). FEBS J 272(16):4270–4283
Speth DR, van Teeseling MC et al (2012) Genomic analysis indicates the presence of an asymmetric bilayer outer membrane in planctomycetes and verrucomicrobia. Front Microbiol 3:304
Stackebrandt E, Wehmeyer U et al (1986) 16S ribosomal RNA- and cell wall analysis of Gemmata obscuriglobus, a new member of the order Planctomycetales. FEMS Microbiol Lett 37(3):289–292
Staley JT, Bouzek H et al (2005) Eukaryotic signature proteins of Prosthecobacter dejongeii and Gemmata sp. Wa-1 as revealed by in silico analysis. FEMS Microbiol Lett 243(1):9–14
Strous M, Pelletier E et al (2006) Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440(7085):790–794
Sutcliffe IC (2010) A phylum level perspective on bacterial cell envelope architecture. Trends Microbiol 18(10):464–470
Teeling H, Lombardot T et al (2004) Evaluation of the phylogenetic position of the planctomycete Rhodopirellula baltica SH 1 by means of concatenated ribosomal protein sequences, DNA-directed RNA polymerase subunit sequences and whole genome trees. Int J Syst Evol Microbiol 54(Pt 3):791–801
Teh AH, Saito JA et al (2011) Hell’s Gate globin I: an acid and thermostable bacterial hemoglobin resembling mammalian neuroglobin. FEBS Lett 585(20):3250–3258
Thomas V, Casson N et al (2006) Criblamydia sequanensis, a new intracellular Chlamydiales isolated from Seine river water using amoebal co-culture. Environ Microbiol 8(12):2125–2135
Thrash JC, Cho JC et al (2010) Genome sequence of Lentisphaera araneosa HTCC2155T, the type species of the order Lentisphaerales in the phylum Lentisphaerae. J Bacteriol 192(11):2938–2939
van Niftrik L, Jetten MS (2012) Anaerobic ammonium-oxidizing bacteria: unique microorganisms with exceptional properties. Microbiol Mol Biol Rev 76(3):585–596
van Niftrik LA, Fuerst JA et al (2004) The anammoxosome: an intracytoplasmic compartment in anammox bacteria. FEMS Microbiol Lett 233(1):7–13
van Niftrik L, Geerts WJ et al (2008) Linking ultrastructure and function in four genera of anaerobic ammonium-oxidizing bacteria: cell plan, glycogen storage, and localization of cytochrome C proteins. J Bacteriol 190(2):708–717
van Niftrik L, van Helden M et al (2010) Intracellular localization of membrane-bound ATPases in the compartmentalized anammox bacterium Candidatus Kuenenia stuttgartiensis. Mol Microbiol 77(3):701–715
van Teeseling MC, Neumann S et al (2013) The anammoxosome organelle is crucial for the energy metabolism of anaerobic ammonium oxidizing bacteria. J Mol Microbiol Biotechnol 23(1–2):104–117
Volkman JK (2003) Sterols in microorganisms. Appl Microbiol Biotechnol 60(5):495–506
Wagner M, Horn M (2006) The Planctomycetes, Verrucomicrobia, Chlamydiae and sister phyla comprise a superphylum with biotechnological and medical relevance. Curr Opin Biotechnol 17(3):241–249
Wang J, Jenkins C et al (2002) Isolation of Gemmata-like and Isosphaera-like planctomycete bacteria from soil and freshwater. Appl Environ Microbiol 68(1):417–422
Weisburg WG, Hatch TP et al (1986) Eubacterial origin of chlamydiae. J Bacteriol 167(2):570–574
Welter-Stahl L, Ojcius DM et al (2006) Stimulation of the cytosolic receptor for peptidoglycan, Nod1, by infection with Chlamydia trachomatis or Chlamydia muridarum. Cell Microbiol 8(6):1047–1057
Wertz JT, Kim E et al (2012) Genomic and physiological characterization of the Verrucomicrobia isolate Diplosphaera colitermitum gen. nov., sp. nov., reveals microaerophily and nitrogen fixation genes. Appl Environ Microbiol 78(5):1544–1555
Wolf YI, Rogozin IB et al (2001) Genome trees constructed using five different approaches suggest new major bacterial clades. BMC Evol Biol 1:8
Yee B, Lafi FF et al (2007) A canonical FtsZ protein in Verrucomicrobium spinosum, a member of the Bacterial phylum Verrucomicrobia that also includes tubulin-producing Prosthecobacter species. BMC Evol Biol 7:37
Yee B, Sagulenko E et al (2011) Making heads or tails of the HU proteins in the planctomycete Gemmata obscuriglobus. Microbiology 157(Pt 7):2012–2021
Yee B, Sagulenko E et al (2012) Electron tomography of the nucleoid of Gemmata obscuriglobus reveals complex liquid crystalline cholesteric structure. Front Microbiol 3:326
Yen TY, Pal S et al (2005) Characterization of the disulfide bonds and free cysteine residues of the Chlamydia trachomatis mouse pneumonitis major outer membrane protein. Biochemistry 44(16):6250–6256
Yildirim S, Yeoman CJ et al (2010) Characterization of the fecal microbiome from non-human wild primates reveals species specific microbial communities. PLoS ONE 5(11):e13963
Yoon J, Matsuo Y et al (2007a) Cerasicoccus arenae gen. nov., sp. nov., a carotenoid-producing marine representative of the family Puniceicoccaceae within the phylum Verrucomicrobia, isolated from marine sand. Int J Syst Evol Microbiol 57(Pt 9):2067–2072
Yoon J, Yasumoto-Hirose M et al (2007b) Coraliomargarita akajimensis gen. nov., sp. nov., a novel member of the phylum Verrucomicrobia isolated from seawater in Japan. Int J Syst Evol Microbiol 57(Pt 5):959–963
Yoon J, Yasumoto-Hirose M et al (2007c) Pelagicoccus mobilis gen. nov., sp. nov., Pelagicoccus albus sp. nov. and Pelagicoccus litoralis sp. nov., three novel members of subdivision 4 within the phylum Verrucomicrobia, isolated from seawater by in situ cultivation. Int J Syst Evol Microbiol 57(Pt 7):1377–1385
Zoetendal EG, Plugge CM et al (2003) Victivallis vadensis gen. nov., sp. nov., a sugar-fermenting anaerobe from human faeces. Int J Syst Evol Microbiol 53(Pt 1):211–215
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuerst, J.A. The PVC superphylum: exceptions to the bacterial definition?. Antonie van Leeuwenhoek 104, 451–466 (2013). https://doi.org/10.1007/s10482-013-9986-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-013-9986-1