Abstract
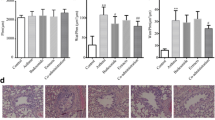
Asthma is a complex chronic inflammatory disease of the airways that involves the activation of many inflammatory and other types of cells. We investigated the effect of low-level laser therapy (LLLT) on allergic asthma in rats and compared its effect with that of the glucocorticoid budesonide. Asthma was induced by challenge and repeated exposure to ovalbumin. Asthmatic rats were then treated with LLLT or budesonide suspension. LLLT at 8 J/cm2 once daily for 21 days could relieve pathological damage and airway inflammation in asthmatic rats. LLLT could decrease the total numbers of cells and eosinophils in bronchoalveolar lavage fluid. LLLT could reduce levels of IL-4 and increase IFN-γ levels in bronchoalveolar lavage fluid and serum, meanwhile reduce serum IgE levels. Flow cytometry assay showed that LLLT can regulate the Th1/Th2 imbalance of asthmatic rats. LLLT had a similar effect to that of budesonide. These findings suggest that the mechanism of LLLT treatment of asthma is by adjustment of Th1/Th2 imbalance. Thus, LLLT could take over some of the effects of budesonide for the treatment of asthma, thereby reducing some of the side effects of budesonide.





Similar content being viewed by others
References
Braman SS (2006) The global burden of asthma. Chest 130:4S–12S. doi:10.1378/chest.130.1_suppl.4S
Basu K, Nair A, Williamson PA, Mukhopadhyay S, Lipworth BJ (2009) Airway and systemic effects of soluble and suspension formulations of nebulized budesonide in asthmatic children. Ann Allergy Asthma Immunol 103:436–441. doi:10.1016/S1081-1206(10)60365-1
Rothers J, Halonen M, Stern DA, Lohman IC, Mobley S, Spangenberg A, Anderson D, Wright AL (2011) Adaptive cytokine production in early life differentially predicts total IgE levels and asthma through age 5 years. J Allergy Clin Immunol 128:397–402. doi:10.1016/j.jaci.2011.04.044
Dubois A, Deruytter N, Adams B, Kanda A, Delbauve S, Fleury S, Torres D, François A, Pétein M, Goldman M, Dombrowicz D, Flamand V (2010) Regulation of Th2 responses and allergic inflammation through bystander activation of CD8+ T lymphocytes in early life. J Immunol 185:884–891. doi:10.4049/jimmunol.0903287
Luzina IG, Lockatell V, Lavania S, Pickering EM, Kang PH, Bashkatova YN, Andreev SM, Atamas SP (2012) Natural production and functional effects of alternatively spliced interleukin-4 protein in asthma. Cytokine 58:20–26. doi:10.1016/j.cyto.2011.12.017
Pires D, Xavier M, Araújo T, Silva JA Jr, Aimbire F, Albertini R (2011) Low-level laser therapy (LLLT; 780 nm) acts differently on mRNA expression of anti- and pro-inflammatory mediators in an experimental model of collagenase-induced tendinitis in rat. Lasers Med Sci 26:85–94. doi:10.1007/s10103-010-0811-z
Aimbire F, Ligeiro de Oliveira AP, Albertini R, Corrêa JC, Ladeira de Campos CB, Lyon JP, Silva JA Jr, Costa MS (2008) Low level laser therapy (LLLT) decreases pulmonary microvascular leakage, neutrophil influx and IL-1beta levels in airway and lung from rat subjected to LPS-induced inflammation. Inflammation 31:189–197. doi:10.1007/s10753-008-9064-4
Takayama S, Tamaoka M, Takayama K, Okayasu K, Tsuchiya K, Miyazaki Y, Sumi Y, Martin JG, Inase N (2011) Synthetic double-stranded RNA enhances airway inflammation and remodelling in a rat model of asthma. Immunology 134:140–150. doi:10.1111/j.1365-2567.2011.03473.x
Chen YH, Wu R, Geng B, Qi YF, Wang PP, Yao WZ, Tang CS (2009) Endogenous hydrogen sulfide reduces airway inflammation and remodeling in a rat model of asthma. Cytokine 45:117–123. doi:10.1016/j.cyto.2008.11.009
Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ, Hong CZ (2012) Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1α (HIF-1α). J Comp Neuro 520:2903–2916. doi:10.1002/cne.23072
Mafra de Lima F, Villaverde AB, Salgado MA, Castro-Faria-Neto HC, Munin E, Albertini R, Aimbire F (2010) Low intensity laser therapy (LILT) in vivo acts on the neutrophils recruitment and chemokines/cytokines levels in a model of acute pulmonary inflammation induced by aerosol of lipopolysaccharide from Escherichia coli in rat. J Photochem Photobiol B 101:271–278. doi:10.1016/j.jphotobiol.2010.07.012
MafradeLima F, Villaverde AB, Albertini R, Corrêa JC, Carvalho RL, Munin E, Araújo T, Silva JA, Aimbire F (2011) Dual effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: action on anti- and pro-inflammatory cytokines. Lasers Surg Med 43:410–420. doi:10.1002/lsm.21053
Aimbire F, Albertini R, Pacheco MT, Castro-Faria-Neto HC, Leonardo PS, Iversen VV, Lopes-Martins RA, Bjordal JM (2006) Low-level laser therapy induces dose-dependent reduction of TNF alpha levels in acute inflammation. Photomed Laser Surg 24:33–37. doi:10.1089/pho.2006.24.33
Mafra de Lima F, Albertini R, Dantas Y, Maia-Filho AL, Santana Cde L, Castro-Faria-Neto HC, França C, Villaverde AB, Aimbire F (2013) Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochem Photobiol 89:179–188. doi:10.1111/j.1751-1097.2012.01214.x
Mafra de Lima F, Vitoretti L, Coelho F, Albertini R, Breithaupt-Faloppa AC, de Lima WT, Aimbire F (2013) Suppressive effect of low-level laser therapy on tracheal hyperresponsiveness and lung inflammation in rat subjected to intestinal ischemia and reperfusion. Lasers Med Sci 28:551–564. doi:10.1007/s10103-012-1088-1
Ma WJ, Li XR, Li YX, Xue ZX, Yin HJ, Ma H (2012) Anti-inflammatory effect of low-level laser therapy on Staphylococcus epidermidis endophthalmitis in rabbits. Lasers Med Sci 27:585–591. doi:10.1007/s10103-011-0991-1
Bensadoun RJ, Nair RG (2012) Low-level laser therapy in the prevention and treatment of cancer therapy-induced mucositis: 2012 state of the art based on literature review and meta-analysis. Curr Opin Oncol 24:363–370. doi:10.1097/CCO.0b013e328352eaa3
Jang H, Lee H (2012) Meta-analysis of pain relief effects by laser irradiation on joint areas. Photomed Laser Surg 30:405–417. doi:10.1089/pho.2012.3240
Careau E, Sirois J, Bissonnette EY (2002) Characterization of lung hyperresponsiveness, inflammation, and alveolar macrophage mediator production in allergy resistant and susceptible rats. Am J Respir Cell Mol Biol 26:579–586. doi:10.1165/ajrcmb.26.5.4737
Abdureyim S, Amat N, Umar A, Upur H, Berke B, Moore N (2011) Anti-inflammatory, immunomodulatory, and heme oxygenase-1 inhibitory activities of ravan napas, a formulation of uighur traditional medicine, in a rat model of allergic asthma. Evid Based Complement Alternat Med. doi:10.1155/2011/725926
Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB (2008) Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy 38:501–511. doi:10.1111/j.1365-2222.2007.02855.x
Silveira MR, Nunes KP, Cara DC, Souza DG, Corrêa A Jr, Teixeira MM, Negrão-Corrêa D (2002) Infection with Strongyloides venezuelensis induces transient airway eosinophilic inflammation, an increase in immunoglobulin E, and hyperresponsiveness in rats. Infect Immun 70:6263–6272. doi:10.1128/IAI.70.11.6263-6272.2002
Cortijo J, Sanz MJ, Iranzo A, Montesinos JL, Nabah YN, Alfón J, Gómez LA, Merlos M, Morcillo EJ (2006) A small molecule, orally active, alpha4beta1/alpha4beta7 dual antagonist reduces leukocyte infiltration and airway hyper-responsiveness in an experimental model of allergic asthma in brown Norway rats. Br J Pharmacol 147:661–670. doi:10.1038/sj.bjp.0706658
Carneiro ER, Xavier RA, De Castro MA, Do Nascimento CM, Silveira VL (2010) Electroacupuncture promotes a decrease in inflammatory response associated with Th1/Th2 cytokines, nitric oxide and leukotriene B4 modulation in experimental asthma. Cytokine 50:335–340. doi:10.1016/j.cyto.2010.01.005
Ohga K, Kuromitsu S, Takezawa R, Numazaki M, Ishikawa J, Nagashima S, Shimizu Y (2008) YM-341619 suppresses the differentiation of spleen T cells into Th2 cells in vitro, eosinophilia, and airway hyperresponsiveness in rat allergic models. Eur J Pharmacol 590:409–416. doi:10.1016/j.ejphar.2008.06.035
Camateros P, Tamaoka M, Hassan M, Marino R, Moisan J, Marion D, Guiot MC, Martin JG, Radzioch D (2007) Chronic asthma-induced airway remodeling is prevented by toll-like receptor-7/8 ligand S28463. Am J Respir Crit Care Med 175:1241–1249. doi:10.1164/rccm.200701-054OC
Motta AC, Vissers JL, Gras R, Van Esch BC, Van Oosterhout AJ, Nawijn MC (2009) GITR signaling potentiates airway hyperresponsiveness by enhancing Th2 cell activity in a mouse model of asthma. Respir Res 10:93. doi:10.1186/1465-9921-10-93
Komai M, Tanaka H, Masuda T, Nagao K, Ishizaki M, Sawada M, Nagai H (2003) Role of Th2 responses in the development of allergen-induced airway remodelling in a murine model of allergic asthma. Br J Pharmacol 138:912–920. doi:10.1038/sj.bjp.0705105
Acknowledgments
This work was supported financially by the Experimental Animal Center of Tianjin Medical University and the Ophthalmic Research Institute of Tianjin Medical University.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
X.-y. Wang and W.-j. Ma contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Wang, Xy., Ma, Wj., Liu, Cs. et al. Effect of low-level laser therapy on allergic asthma in rats. Lasers Med Sci 29, 1043–1050 (2014). https://doi.org/10.1007/s10103-013-1456-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-013-1456-5