Abstract
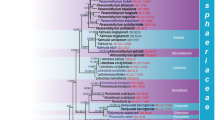
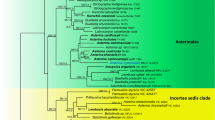
Ambispora, the only genus in Ambisporaceae and one of three deeply rooted families in Archaeosporales, Glomeromycetes, is amended. Analysis of the morphology of specimens from types and living cultures and 28S ribosomal DNA (rDNA; LSU) sequences resulted in two major changes that redefined Ambispora to include only species with the potential for spore dimorphism (acaulosporoid and glomoid). First, species described as producing only glomoid spores (Ambispora leptoticha, Ambispora fecundispora, and Ambispora callosa), only acaulosporoid spores (Ambispora jimgerdemannii), or both spore morphotypes (Ambispora appendicula) were synonymized with a redefined dimorphic species, A. leptoticha. LSU sequences and more conserved SSU gene data indicated little divergence between genotypes formerly classified as separate species. Second, Ambispora fennica was synonymized with Ambispora gerdemannii based on morphological and LSU sequence variation equivalent to that measured in the sister clade A. leptoticha. With this analysis, Ambispora was reduced to three species: A. leptoticha, A. gerdemannii, and Ambispora granatensis. Morphological and molecular characters were given equal treatment in this study, as each data set informed and clarified grouping and ranking decisions. The two inner layers of the acaulosporoid spore wall were the only structural characters uniquely defining each of these three species; all other characters were shared. Phenotypes of glomoid spores were indistinguishable between species, and thus were informative only at the genus level. Distinct subclade structure of the LSU gene tree suggests fixation of discrete variants typical of clonal reproduction and possible retention of polymorphisms in rDNA repeats, so that not all discrete genetic variants are indicative of speciation.







Similar content being viewed by others
References
Ames RN, Linderman RG (1976) Acaulospora trappei sp. nov. Mycotaxon 3:565–569
Davis JI, Nixon KC (1992) Populations, genetic variation, and the delimitation of phylogenetic species. Syst Biol 41:421–435
Edgar RC (2004) MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5:1–19
Gautam SP, Patel US (2007) Rhizoendomutualmycota (REMM): a new phylum for the farmers’ friend number one. In: Tiwari M, Sati SC (eds) The Mycorrhizae: diversity, ecology and applications. Daya Pub. House, Delhi, pp pp. 1–pp. 13
Gerdemann JW, Trappe J (1974) The Endogonaceae in the Pacific Northwest. Mycol Mem 5:1–76
Heath TA, Hedtke SM, Hillis DM (2008) Taxon sampling and the accuracy of phylogenetic analyses. J Syst Evol 46:239–257
Hillis DM (1987) Molecular versus morphological approaches to systematics. Ann Rev Ecol Syst 18:23–42
Kaonongbua W, Morton JB, Bever JD (2010) Taxonomic revision transferring species in Kuklospora to Acaulospora (Glomeromycota) and a description of Acaulospora colliculosa sp. nov. from field collected spores. Mycologia 102:1497–1509
Kojima T, Sawaki H, Saito M (2004) Detection of arbuscular mycorrhizal fungi, Archaeospora leptoticha, and related species colonizing plant roots by specific PCR primer. Soil Sci Plant Nutr 50:95–101
Koske RE, Tessier B (1983) A convenient, permanent slide mounting medium. Mycol Soc Amer Newslett 34:59
Krüger M, Krüger C, Walker C, Stockinger H, Schüssler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984
Morton JB (1990) Evolutionary relationships among arbuscular mycorrhizal fungi in the Endogonaceae. Mycologia 82:192–207
Morton JB (1995) Taxonomic and phylogenetic divergence among five Scutellospora species (Glomales, Zygomycetes) based on comparative developmental sequences. Mycologia 87:127–137
Morton JB (2009) Reconciliation of conflicting morphological and rRNA gene phylogenies of fungi in Glomeromycota based on underlying patterns and processes. In: Azcon-Aguilar C, Barea JM, Gianinazzi S, Gianinazzi-Pearson V (eds) Mycorrhizas—functional processes and ecological impact. Springer-Verlag, Berlin, pp 137–154
Morton JB, Msiska Z (2010a) Phylogenies from genetic and morphological characters do not support a revision of Gigasporaceae (Glomeromycota) into four families and five genera. Mycorrhiza 19:501–513
Morton JB, Msiska Z (2010b) Ontogeny and phylogeny of Scutellospora heterogama mutant, with implications for morphological recognition of species in Glomeromycota. Fungal Biol 114:410–420
Morton JB, Redecker D (2001) Two new families of Glomales, Archaeosporaceae and Paraglomeraceae, with two new genera Archaeospora and Paraglomus, based on concordant molecular and morphological characters. Mycologia 93:181–195
Morton JB, Bentivenga SP, Wheeler WW (1993) Germ plasm in the International Collection of Arbuscular and Vesicular-arbuscular Mycorrhizal Fungi (INVAM) and procedures for culture and development, documentation and storage. Mycotaxon 48:491–528
Morton JB, Bever JD, Pfleger FL (1997) Taxonomy of Acaulospora gerdemannii and Glomus leptotichum, synamorphs of an arbuscular mycorrhizal fungus in Glomales. Mycol Res 101:625–631
Msiska Z, Morton JB (2009) Phylogenetic analysis of the Glomeromycota by a partial β-tubulin gene. Mycorrhiza 19:247–254
Nei M, Kumar S (2000) Molecular Evolution and Phylogenetics. New York, Oxford University Press
Nicolson TH, Schenck NC (1979) Endogonaceous mycorrhizal endophytes in Florida. Mycologia 71:178–198
Oehl F, Sieverding E, Palenzuela J, Ineichen K, da Silva GA (2011) Advances in Glomeromycota taxonomy and classification. IMA Fungus 2:191–199
Oehl F, Castillo C, Schneider D, Säle V, Sieverding E (2012) Ambispora reticulata, a new species in the Glomeromycota from mountainous areas in Switzerland and Chile. J Appl Bot Food Qual 85:129–133
Palenzuela J, Barea J, Ferrol N, Oehl F (2011) Ambispora granatensis, a new arbuscular mycorrhizal fungus, associated with Asparagus officinalis in Andalucia (Spain). Mycologia 103:333–340
Pollock DD, Zwickl DJ, McGuire JA, Hillis DM (2002) Increased taxon sampling is advantageous for phylogenetic inference. Syst Biol 51:664–671
Redecker D, Raab P (2006) Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): Recent developments and new gene markers. Mycologia 98:885–895
Redecker D, Morton JB, Bruns TD (2000) Ancestral lineages of arbuscular mycorrhizal fungi (Glomales). Mol Phylogenet Evol 14:276–284
Redecker D, Schüßler A, Stockinger H, Stürmer SL, Morton JB, Walker C (2013) An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23:515–531
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model across a large model space. Syst Biol 61:539--542
Rose S, Daniels BA, Trappe JM (1979) Glomus gerdemannii sp. nov. Mycotaxon 8:297–301
Sawaki H, Sugawara K, Saito M (1998) Phylogenetic position of an arbuscular mycorrhizal fungus, Acaulospora gerdemannii, and its synamorph Glomus leptotichum, based on 18S rRNA gene sequence. Mycoscience 39:477–480
Schenck NC, Smith GS (1982) Additional new and unreported species of mycorrhizal fungi (Endogonaceae) from Florida. Mycologia 74:77–92
Schenck NC, Spain JL, Sieverding E, Howeler RH (1984) Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 76:685–699
Schüßler A (2002) Molecular phylogeny, taxonomy, and evolution of Geosiphon pyriformis and arbuscular mycorrhizal fungi. Plant Soil 244:75–83
Schüßler A, Schwarzott D, Walker C (2001) A new fungal phylum, the Glomeromycota: evolution and phylogeny. Mycol Res 105:1413–1421
Sieverding E (1988) Two new species of vesicular arbuscular mycorrhizal fungi in the Endogonaceae from tropical highlands of Africa. Angew Botanik 62:373–380
Sieverding E, Toro S (1987) Entrophospora schenckii, a new species in the Endogonaceae from Colombia. Mycotaxon 28:209–295
Spain JL, Sieverding E, Oehl F (2006) Appendicispora: a new genus in the arbuscular mycorrhiza-forming Glomeromycetes, with a discussion of the genus Archaeospora. Mycotaxon 97:163–182
Stockinger H, Krüger M, Schüßler A (2010) DNA barcoding of arbuscular mycorrhizal fungi. New Phytol 187:461–474
Stürmer SL, Morton JB (1997) Developmental patterns defining morphological characters in spores of species in Glomus (Glomales, Zygomycetes). Mycologia 89:72–81
Stürmer SL, Morton JB (1999) Scutellospora rubra, a new arbuscular mycorrhizal species from Brazil. Mycol Res 103:949–954
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetic analyses version 6.0. Mol Biol Evol 30:2725–2729
Trouvelet S, van Tuinen D, Hijri M, Gianinazzi-Pearson V (1999) Visualization of ribosomal DNA loci in spore interphasic nuclei of glomalean fungi by fluorescence in situ hybridization. Mycorrhiza 8:203–206
van Tuinen D, Jacquot E, Zhao B, Gollotte A, Gianinazzi-Pearson V (1998) Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol Ecol 7:879–887
vanKuren NW, den Bakker HC, Morton JB, Pawlowska TE (2013) Ribosomal RNA gene diversity, effective population size, and evolutionary longevity in asexual Glomeromycota. Evolution 67:207–224
Walker C (2008) Ambispora and Ambisporaceae resurrected. Mycol Res 112:297–298
Walker C, Vestberg M, Demircik F, Stockinger H, Saito M, Sawaki H, Nishmura I, Schüßler A (2007a) Molecular phylogeny and new taxa in the Archaeosporales (Glomeromycota): Ambispora fennica gen. sp. nov., Ambisporaceae fam. nov., and emendation of Archaeospora and Archaeosporaceae. Mycol Res 111:137–153
Walker C, Vestberg M, Schüßler A (2007b) Nomenclatural clarification in Glomeromycota. Mycol Res 111:253–256
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: A guide to methods and applications. Academic Press, San Diego, pp 315–322
Acknowledgments
The authors thank Bill Wheeler for assistance in growing and processing AMF cultures. We also appreciate the assistance of Dr. Donna Ford-Werntz (curator of the WVU Herbarium), Dr. Richard Halse (curator Oregon State University Herbarium), Reinhard Berndt (curator of fungus collections herbaria Z + ZT Zürich, Switzerland), Janus Blaszkowski, Jim Bever, and Wittaya Kaonongbua for providing herbarium and other specimens. Funding support was provided by National Science Foundation grants DBI0650735 and DEB0649341 to Joseph Morton.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bills, R.J., Morton, J.B. A combination of morphology and 28S rRNA gene sequences provide grouping and ranking criteria to merge eight into three Ambispora species (Ambisporaceae, Glomeromycota). Mycorrhiza 25, 485–498 (2015). https://doi.org/10.1007/s00572-015-0626-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0626-7