Abstract
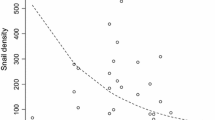
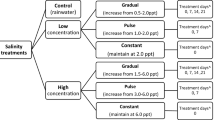
With the incidence of emerging infectious diseases on the rise, it is becoming increasingly important to identify refuge areas that protect hosts from pathogens and therefore prevent population declines. For the chytrid fungus Batrachochytrium dendrobatidis, temperature and humidity refuge areas for amphibian hosts exist but are difficult to manipulate. Other environmental features that may affect the outcome of infection include water quality, drying regimes, abundance of alternate hosts and isolation from other hosts. We identified relationships between water bodies with these features and infection levels in the free-living hosts inhabiting them. Where significant relationships were identified, we used a series of controlled experiments to test for causation. Infection loads were negatively correlated with the salt concentration of the aquatic habitat and the degree of water level fluctuation and positively correlated with fish abundance. However, only the relationship with salt was confirmed experimentally. Free-living hosts inhabiting water bodies with mean salinities of up to 3.5 ppt had lower infection loads than those exposed to less salt. The experiment confirmed that exposure to sodium chloride concentrations >2 ppt significantly reduced host infection loads compared to no exposure (0 ppt). These results suggest that the exposure of amphibians to salt concentrations found naturally in lentic habitats may be responsible for the persistence of some susceptible species in the presence of B. dendrobatidis. By manipulating the salinity of water bodies, it may be possible to create refuges for declining amphibians, thus allowing them to be reintroduced to their former ranges.




Similar content being viewed by others
References
Atkinson C, Dusek R, Woods K, Iko W (2000) Pathogenicity of avian malaria in experimentally-infected Hawaii Amakihi. J Wildl Dis 36:197–204
Australian Govt. Bureau of Meteorology (2007) Homepage. http://www.bom.gov.au
Becker CG, Zamudio KR (2011) Tropical amphibian populations experience higher disease risk in natural habitats. Proc Natl Acad Sci USA 108:9893–9898. doi:10.1073/pnas.1014497108
Becker CG, Rodriguez D, Longo AV, Talaba AL, Zamudio KR (2012) Disease risk in temperate amphibian populations is higher at closed-canopy sites. PLoS One 7:e48205. doi:10.1371/journal.pone.0048205
Berger L et al (1998) Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA 95:9031–9036
Berger L et al (2004) Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Aust Vet J 82:434–440
Blomberg A, Adler L (1993) Tolerence of fungi to NaCl. In: Jennings D (ed) Stress tolerance of fungi. Marcel Dekker, New York, pp 209–231
Bosch J, Rincon PA (2008) Chytridiomycosis-mediated expansion of Bufo bufo in a montane area of Central Spain: an indirect effect of the disease. Divers Distrib 14:637–643. doi:10.1111/j.1472-4642.2007.00461.x
Boyle DB, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org 60:141–148
Bramwell R (2011) Do salinity and pH help protect natterjack toads from chytridiomycosis, a disease caused by the amphibian fungus Batrachochytrium dendrobatidis (B.d.)? Masters thesis. Imperial College, London
Briggs CJ, Knapp RA, Vredenburg VT (2010) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci USA 107:9695–9700
Carey C, Cohen N, Rollins-Smith L (1999) Amphibian declines: an immunological perspective. Dev Comp Immunol 23:459–472
Carey C et al (2006) Experimental exposures of boreal toads (Bufo boreas) to a pathogenic chytrid fungus (Batrachochytrium dendrobatidis). EcoHealth 3:5–21
Carroll C, Dunk JR, Moilanen A (2010) Optimizing resiliency of reserve networks to climate change: multispecies conservation planning in the Pacific Northwest USA. Glob Change Biol 16:891–904. doi:10.1111/j.1365-2486.2009.01965.x
Costa J, Peterson AT (2012) Ecological niche modeling as a tool for understanding distributions and interactions of vectors, hosts, and etiologic agents of chagas disease. In: Mylonakis E, Ausubel FM, Gilmore M, Casadevall A (eds) Recent advances on model hosts, vol 710. Springer, Berlin, pp 59–70
DiGiacomo RF, Koepsell TD (1986) Sampling for detection of infection or disease in animal populations. J Am Vet Med Assoc 189:22–23
Dobson A, Foufopoulos J (2001) Emerging infectious pathogens of wildlife. Philosop Trans Biol Sci 356:1001–1012
Garmyn A, Van Rooij P, Pasmans F, Hellebuyck T, Van Den Broeck W, Haesebrouck F, Martel A (2012) Waterfowl: potential environmental reservoirs of the chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 7:e35038
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Hamer AJ (2008) Movement patterns of adult green and golden bell frogs Litoria aurea and the implications for conservation management. J Herpetol 42:397–407
Hanski I (1999) Metapopulation ecology. Oxford University Press, New York
Heard GW, Scroggie MP, Clemann N, Ramsey DSL (2014) Wetland characteristics influence disease risk for a threatened amphibian. Ecol Appl 24:650–662. doi:10.1890/13-0389.1
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol Monogr 63:77–101. doi:10.2307/2937124
Johnson ML, Speare R (2003) Survival of Batrachochytrium dendrobatidis in water: qarantine and disease control implications. Emerg Infect Dis 9:922–925
Johnson ML, Berger L, Philips L, Speare R (2003) Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Org 57:255–260
Kaushal SS et al (2005) Increased salinization of fresh water in the northeastern United States. Proc Natl Acad Sci USA 102:13517–13520
Kearney BD, Byrne PG, Reina RD (2012) Larval tolerance to salinity in three species of Australian anuran: an indication of saline specialisation in Litoria aurea. PLoS ONE. doi:10.1371/journal.pone.0043427
Lane A, Burgin S (2008) Comparison of frog assemblages between urban and non-urban habitats in the upper Blue Mountains of Australia. Freshwat Biol 53:2484–2493. doi:10.1111/j.1365-2427.2008.02068.x
Levinton J, Doall M, Ralston D, Starke A, Allam B (2011) Climate change, precipitation and impacts on an estuarine refuge from disease. PLoS ONE. doi:10.1371/journal.pone.0018849
Mahony M (1999) Review of the declines and disappearances within the bell frog species group (Litoria aurea species group) in Australia. In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra, pp 81–93
Mahony MJ, Clulow J, Browne RK, Pomering M (1999) Declines and disappearances of frogs: risk assessment and contingency strategies. In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra
McConnell TH (2007) The nature of disease. Lippincott, Williams and Wilkins, Baltimore
McDonald K, Alford R (1999) A review of declining frogs in Northern Queensland. In: Campbell A (ed) Declines and disappearances of Australian frogs. Environment Australia, Canberra, pp 14–22
McDowall RM (1980) Freshwater fishes of south-eastern Australia. Reed, Terrey Hills
McMahon TA, Brannelly LA, Chatfield MWH, Johnson PTJ, Joseph MB, McKenzie VJ, Richards-Zawacki CL, Venesky MD, Rohr JR (2012) Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection. Proc Natl Acad Sci 110:210–215
Minting PJ (2012) An investigation into the effects of Batrachochytrium dendrobatidis (Bd) on natterjack toad (Bufo calamita) populations in the UK. Ph.D. thesis. The University of Sussex, Brighton
Moyle PB, Cech JJJ (2000) Fishes: an introduction to ichthyology, 4th edn. Prentice Hall, Sydney
Muths E, Pilliod DS, Livo LJ (2008) Distribution and environmental limitations of an amphibian pathogen in the Rocky Mountains USA. Biol Conserv 141:1484–1492. doi:10.1016/j.biocon.2008.03.011
Orrock JL, Holt RD, Baskett ML (2010) Refuge-mediated apparent competition in plant–consumer interactions. Ecol Lett 13:11–20. doi:10.1111/j.1461-0248.2009.01412.x
Parris MJ, Beaudoin JG (2004) Chytridiomycosis impacts predator–prey interactions in larval amphibian communities. Oecologia 140:626–632
Parris MJ, Cornelius TO (2004) Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology 85:3385–3395
Peterson AT (2006) Ecologic niche modeling and spatial patterns of disease transmission. Emerg Infect Dis 12:1822–1826
Pinkard EA, Kriticos DJ, Wardlaw TJ, Carnegie AJ, Leriche A (2010) Estimating the spatio-temporal risk of disease epidemics using a bioclimatic niche model. Ecol Model 221:2828–2838. doi:10.1016/j.ecolmodel.2010.08.017
Piotrowski JS, Annis SL, Longcore JE (2004) Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 96:9–15
Pitman MG (2002) Global impact of salinity and agricultural ecosystems. In: Lauchli A, Luttge U (eds) Salinity: environment–plants–molecules. Kluwer, Dordrecht, pp 3–20
Puschendorf R et al (2009) Distribution models for the amphibian chytrid Batrachochytrium dendrobatidis in Costa Rica: proposing climatic refuges as a conservation tool. Divers Distrib 15:401–408. doi:10.1111/j.1472-4642.2008.00548.x
Puschendorf R et al (2011) Environmental refuge from disease-driven amphibian extinction (Refugio ambiental para la extinción de anfibios causada por enfermedad). Conserv Biol 25:956–964. doi:10.1111/j.1523-1739.2011.01728.x
Pyke GH (2008) Plague minnow or mosquito fish? A review of the biology and impacts of introduced species. Annu Rev Ecol Evol Syst 39:171–191
Raffel T, Michel P, Sites E, Rohr J (2010) What drives chytrid infections in newt populations? Associations with substrate, temperature, and shade. EcoHealth 7:526–536. doi:10.1007/s10393-010-0358-2
Rodder D, Veith M, Lotters S (2008) Environmental gradients explaining the prevalence and intensity of infection with the amphibian chytrid fungus: the host’s perspective. Anim Conserv 11:513–517. doi:10.1111/j.1469-1795.2008.00210.x
Ron SR (2005) Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 37:209–221
Segerström U, Bradshaw R, Hörnberg G, Bohlin E (1994) Disturbance history of a swamp forest refuge in northern Sweden. Biol Conserv 68:189–196. doi:10.1016/0006-3207(94)90350-6
Shapard EJ, Moss AS, San Francisco MJ (2012) Batrachochytrium dendrobatidis can Infect and cause mortality in the Nematode Caenorhabditis elegans. Mycopathologia 173:121–126
Skerratt LF et al (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Speare R, Berger L (2005) Chytridiomycosis status of wild amphibians in Australia. http://www.jcu.edu.au/school/phtm/PHTM/frogs/chy-au-status.htm. Accessed 14/3/12
Springer YP, Hardcastle BA, Gilbert GS (2007) Soil calcium and plant disease in serpentine ecosystems: a test of the pathogen refuge hypothesis. Oecologia 151:10–21. doi:10.1007/s00442-006-0566-1
Stevenson LA, Alford RA, Bell SC, Roznik EA, Berger L, Pike DA (2013) Variation in thermal performance of a widespread pathogen, the amphibian chytrid fungus Batrachochytrium dendrobatidis. PLoS ONE 8:e73830. doi:10.1371/journal.pone.0073830
Stockwell MP, Clulow S, Clulow J, Mahony M (2008) The impact of the amphibian chytrid fungus Batrachochytrium dendrobatidis on a green and golden bell frog Litoria aurea reintroduction program at the Hunter Wetlands Centre Australia in the Hunter Region of NSW. Aust Zool 34:379–386
Stockwell MP, Clulow J, Mahony MJ (2010) Host species determines whether infection load increases beyond disease-causing thresholds following exposure to the amphibian chytrid fungus. Anim Conserv 13(Suppl 1):62–71
Stockwell MP, Clulow J, Mahony MJ (2012) Sodium chloride inhibits the growth and infective capacity of the amphibian chytrid fungus and increases host survival rates. PLoS ONE 7:e36942. doi:10.1371/journal.pone.0036942
Threlfall CG, Jolley DF, Evershed N, Goldingay RL, Buttemer WA (2008) Do green and golden bell frogs Litoria aurea occupy habitats with fungicidal properties? Aust Zool 34:350–360
Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ (2010) Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA 107:9689–9694
Wobeser G (2002) Disease management strategies for wildlife. Rev Sci Tech Off Int Epizoot 21:159–178
Wobeser G (2007) Disease management through environmental modification. Disease in wild animals. Springer, Berlin, pp 271–290
Wright KM, Whitaker BR (2001) Pharmacotherapeutics. In: Wright KM, Whitaker BR (eds) Amphibian medicine and captive husbandry. Krieger, Malabar, pp 309–319
Acknowledgments
We would like to acknowledge the Australian Animal Health Laboratory for training in real-time PCR and providing chytrid isolates. We thank Evan Pickett, Riona Tindal, Dale Bond and Tegan Hunter for assistance with data collection. This work was funded by the Port Waratah Coal Service through the Kooragang Wetland Rehabilitation Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ross Andrew Alford.
Rights and permissions
About this article
Cite this article
Stockwell, M.P., Clulow, J. & Mahony, M.J. Evidence of a salt refuge: chytrid infection loads are suppressed in hosts exposed to salt. Oecologia 177, 901–910 (2015). https://doi.org/10.1007/s00442-014-3157-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-014-3157-6