Abstract
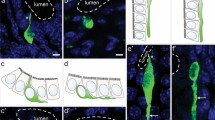
Various digestive and enteroendocrine signaling processes are constantly being adapted to the chemical composition and quantity of the chyme contained in the diverse compartments of the gastrointestinal tract. The chemosensory monitoring that underlies the adaptive capacity of the gut is thought to be performed by so-called brush cells that share morphological and molecular features with gustatory sensory cells. A substantial population of brush cells is localized in the gastric mucosa. However, no chemosensory receptors have been found to be expressed in these cells so far, challenging the concept that they serve a chemosensory function. The canonical chemoreceptors for the detection of macronutrients are taste receptors belonging to the T1R family; these have been identified in several tissues in addition to the gustatory system including the small intestine. We demonstrate the expression of the T1R subtype T1R3, which is essential for the detection of both sugars and amino acids in the gustatory system, in two distinct cell populations of the gastric mucosa. One population corresponds to open-type brush cells, emphasizing the notion that they are a chemosensory cell type; T1R3 immunoreactivity in these cells is restricted to the apical cell pole, which might provide the basis for the detection of luminal macronutrient compounds. The second gastric T1R3-positive population consists of closed-type endocrine cells that produce ghrelin. This finding suggests that ghrelin-releasing cells, which lack access to the stomach lumen, might receive chemosensory input from macronutrients in the circulation via T1R3.







Similar content being viewed by others
References
Bezençon C, Coutre J le, Damak S (2007) Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses 32:41–49
Cummings DE, Overduin J (2007) Gastrointestinal regulation of food intake. J Clin Invest 117:13–23
Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719
Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M (2000) Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 141:4255–4261
Dornonville de la Cour C, Björkqvist M, Sandvik AK, Bakke I, Zhao CM, Chen D, Håkanson R (2001) A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept 99:141–150
Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP (2005) Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33:302–305
Foster-Schubert KE, Overduin J, Prudom CE, Liu J, Callahan HS, Gaylinn BD, Thorner MO, Cummings DE (2008) Acyl and total ghrelin are suppressed strongly by ingested proteins, weakly by lipids, and biphasically by carbohydrates. J Clin Endocrinol Metab 93:1971–1979
Gomez G, Englander EW, Greeley GH Jr (2004) Nutrient inhibition of ghrelin secretion in the fasted rat. Regul Pept 117:33–36
Hass N, Schwarzenbacher K, Breer H (2007) A cluster of gustducin-expressing cells in the mouse stomach associated with two distinct populations of enteroendocrine cells. Histochem Cell Biol 128:457–471
Höfer D, Drenckhahn D (1992) Identification of brush cells in the alimentary and respiratory system by antibodies to villin and fimbrin. Histochemistry 98:237–242
Höfer D, Drenckhahn D (1996) Cytoskeletal markers allowing discrimination between brush cells and other epithelial cells of the gut including enteroendocrine cells. Histochem Cell Biol 105:405–412
Höfer D, Püschel B, Drenckhahn D (1996) Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 93:6631–6634
Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS (1999) Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell 96:541–551
Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 104:15069–15074
Jeon TI, Zhu B, Larson JL, Osborne TF (2008) SREBP-2 regulates gut peptide secretion through intestinal bitter taste receptor signaling in mice. J Clin Invest 118:3693–3700
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660
Kruger DF, Martin CL, Sadler CE (2006) New insights into glucose regulation. Diabetes Educ 32:221–228
Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E (2002) Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99:4692–4696
Luciano L, Reale E (1990) Brush cells of the mouse gallbladder. A correlative light- and electron-microscopical study. Cell Tissue Res 262:339–349
Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, Maillet EL, Ninomiya Y, Mosinger B, Shirazi-Beechey SP (2007) T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 104:15075–15080
McCowen KC, Maykel JA, Bistrian BR, Ling PR (2002) Circulating ghrelin concentrations are lowered by intravenous glucose or hyperinsulinemic euglycemic conditions in rodents. J Endocrinol 175:R7–R11
Murray CD, Roux CW le, Gouveia C, Bassett P, Ghatei MA, Bloom SR, Emmanuel AV, Gabe SM (2006) The effect of different macronutrient infusions on appetite, ghrelin and peptide YY in parenterally fed patients. Clin Nutr 25:626–633
Nakagawa Y, Nagasawa M, Yamada S, Hara A, Mogami H, Nikolaev VO, Lohse MJ, Shigemura N, Ninomiya Y, Kojima I (2009) Sweet taste receptor expressed in pancreatic beta-cells activates the calcium and cyclic AMP signaling systems and stimulates insulin secretion. PLoS ONE 4:e5106
Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (2001) Mammalian sweet taste receptors. Cell 106:381–390
Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS (2002) An amino-acid taste receptor. Nature 416:199–202
Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE (2005) Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 146:845–850
Prodam F, Me E, Riganti F, Gramaglia E, Bellone S, Baldelli R, Rapa A, Lely AJ van der, Bona G, Ghigo E, Broglio F (2006) The nutritional control of ghrelin secretion in humans: the effects of enteral vs. parenteral nutrition. Eur J Nutr 45:399–405
Qader SS, Salehi A, Håkanson R, Lundquist I, Ekelund M (2005) Long-term infusion of nutrients (total parenteral nutrition) suppresses circulating ghrelin in food-deprived rats. Regul Pept 131:82–88
Ren X, Zhou L, Terwilliger R, Newton SS, Araujo IE de (2009) Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr Neurosci 3:12
Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E (2006) Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291:G792–G802
Sakata I, Nakamura K, Yamazaki M, Matsubara M, Hayashi Y, Kangawa K, Sakai T (2002) Ghrelin-producing cells exist as two types of cells, closed- and opened-type cells, in the rat gastrointestinal tract. Peptides 23:531–536
Shrestha YB, Wickwire K, Giraudo SQ (2009) Direct effects of nutrients, acetylcholine, CCK, and insulin on ghrelin release from the isolated stomachs of rats. Peptides 30:1187–1191
Trier JS, Allan CH, Marcial MA, Madara JL (1987) Structural features of the apical and tubulovesicular membranes of rodent small intestinal tuft cells. Anat Rec 219:69–77
Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C (2001) Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest 24:RC19–RC21
Wattel W, Geuze JJ (1978) The cells of the rat gastric groove and cardia. An ultrastructural and carbohydrate histochemical study, with special reference to the fibrillovesicular cells. Cell Tissue Res 186:375–391
Williams DL, Cummings DE, Grill HJ, Kaplan JM (2003) Meal-related ghrelin suppression requires postgastric feedback. Endocrinology 144:2765–2767
Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E (2002) Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 99:2392–2397
Acknowledgements
The authors thank Kerstin Bach and Anne Ullrich for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Kompetenznetz Adipositas (Competence Network of Obesity; research focus: Obesity and the GI tract) funded by the Federal Ministry of Education and Research (no. 01GI0843). Nicole Hass is a recipient of a Peter und Traudl Engelhorn Stiftung scholarship.
Rights and permissions
About this article
Cite this article
Hass, N., Schwarzenbacher, K. & Breer, H. T1R3 is expressed in brush cells and ghrelin-producing cells of murine stomach. Cell Tissue Res 339, 493–504 (2010). https://doi.org/10.1007/s00441-009-0907-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0907-6