Abstract
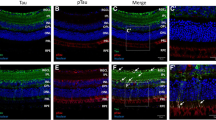
rTg4510 transgenic (TG) mice overexpress mutant (P301L) human tau protein. We have compared the dorsal premotor cortex of TG mice versus non-transgenic (NT) mice at 4, 9, and 13 months of age, using light (LM) and electron microscopy (EM). LM assessment shows that cortical thickness in TG mice is reduced by almost 50% from 4 to 13 months of age, while at the same time layer I thickness is reduced by 80%, with most of the cortical thinning occurring between 4 and 9 months. In TG mice, spherical, empty vacuoles, up to 60 μm in diameter, become increasingly abundant with age and by 9 months, pyramidal and non-pyramidal neurons with large intracellular tangles of tau protein are common throughout the cortex. These tangles occur in the perikarya; we have not observed them entering into cellular processes, nor have we observed ghost tangles in the intercellular matrix. In TG mice, nerve fiber pathology is widespread by 13 months, and split myelin sheaths, ballooned sheaths, and swollen axons containing mitochondrial aggregations are all common. Astrocytes become increasingly filled with glial filaments as TG mice age, and microglial cells almost always contain phagocytic inclusions. However, no glial cells are seen to contain tau in their cytoplasm. These observations add to the base of knowledge available on this commonly employed model of tauopathy.










Similar content being viewed by others
References
Adams SJ, Crook RJ, Deture M, Randle SJ, Innes AE, Yu XZ et al (2009) Overexpression of wild-type murine tau results in progressive tauopathy and neurodegeneration. Am J Pathol 175:1598–1609
Ashe KH, Zahs KR (2010) Probing the biology of Alzheimer’s disease in mice. Neuron 66:631–645
Baloyannis SJ (2005) Morphological and morphometric alterations of Cajal–Retzius cells in early cases of Alzheimer’s disease: a golgi and electron microscope study. Int J Neurosci 115:965–980
Bird TD, Nochlin D, Poorkaj P, Cherrier M, Kaye J, Payami H et al (1999) A clinical pathological comparison of three families with frontotemporal dementia and identical mutations in the tau gene (P301L). Brain 122(Pt 4):741–756
de Calignon A, Spires-Jones TL, Pitstick R, Carlson GA, Hyman BT (2009) Tangle-bearing neurons survive despite disruption of membrane integrity in a mouse model of tauopathy. J Neuropathol Exp Neurol 68:757–761
de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL et al (2010) Caspase activation precedes and leads to tangles. Nature 464:1201–1204
Desai MK, Sudol KL, Janelsins MC, Mastrangelo MA, Frazer ME, Bowers WJ (2009) Triple-transgenic Alzheimer’s disease mice exhibit region-specific abnormalities in brain myelination patterns prior to appearance of amyloid and tau pathology. Glia 57:54–65
Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K et al (2007) Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain 130:1159–1166
Duff K, Suleman F (2004) Transgenic mouse models of Alzheimer’s disease: how useful have they been for therapeutic development? Brief Funct Genomic Proteomic 3:47–59
Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL et al (2003) Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA 100:10032–10037
Hasegawa M, Smith MJ, Goedert M (1998) Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett 437:207–210
Higuchi M, Ishihara T, Zhang B, Hong M, Andreadis A, Trojanowski J et al (2002) Transgenic mouse model of tauopathies with glial pathology and nervous system degeneration. Neuron 35:433–446
Higuchi M, Zhang B, Forman MS, Yoshiyama Y, Trojanowski JQ, Lee VM (2005) Axonal degeneration induced by targeted expression of mutant human tau in oligodendrocytes of transgenic mice that model glial tauopathies. J Neurosci 25:9434–9443
Hong M, Zhukareva V, Vogelsberg-Ragaglia V, Wszolek Z, Reed L, Miller BI et al (1998) Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 282:1914–1917
Im K, Lee JM, Seo SW, Yoon U, Kim ST, Kim YH et al (2008) Variations in cortical thickness with dementia severity in Alzheimer’s disease. Neurosci Lett 436:227–231
Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I (2009) Mechanisms of tau-induced neurodegeneration. Acta Neuropathol 118:53–69
Iseki E, Yamamoto R, Murayama N, Minegishi M, Togo T, Katsuse O et al (2006) Immunohistochemical investigation of neurofibrillary tangles and their tau isoforms in brains of limbic neurofibrillary tangle dementia. Neurosci Lett 405:29–33
Janus C (2008) Conditionally inducible tau mice—designing a better mouse model of neurodegenerative diseases. Genes Brain Behav 7(Suppl 1):12–27
Leroy K, Bretteville A, Schindowski K, Gilissen E, Authelet M, De Decker R et al (2007) Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am J Pathol 171:976–992
Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M et al (2000) Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25:402–405
Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G et al (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487–1491
Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW (2003a) Filamentous tau in oligodendrocytes and astrocytes of transgenic mice expressing the human tau isoform with the P301L mutation. Am J Pathol 162:213–218
Lin WL, Lewis J, Yen SH, Hutton M, Dickson DW (2003b) Ultrastructural neuronal pathology in transgenic mice expressing mutant (P301L) human tau. J Neurocytol 32:1091–1105
Lin WL, Zehr C, Lewis J, Hutton M, Yen SH, Dickson DW (2005) Progressive white matter pathology in the spinal cord of transgenic mice expressing mutant (P301L) human tau. J Neurocytol 34:397–410
Mirra SS, Murrell JR, Gearing M, Spillantini MG, Goedert M, Crowther RA et al (1999) Tau pathology in a family with dementia and a P301L mutation in tau. J Neuropathol Exp Neurol 58:335–345
Nasreddine ZS, Loginov M, Clark LN, Lamarche J, Miller BL, Lamontagne A et al (1999) From genotype to phenotype: a clinical pathological, and biochemical investigation of frontotemporal dementia and parkinsonism (FTDP-17) caused by the P301L tau mutation. Ann Neurol 45:704–715
Perl DP (2010) Neuropathology of Alzheimer’s disease. Mt Sinai J Med 77:32–42
Probst A, Gotz J, Wiederhold KH, Tolnay M, Mistl C, Jaton AL et al (2000) Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol 99:469–481
Ramalho RM, Viana RJ, Castro RE, Steer CJ, Low WC, Rodrigues CM (2008) Apoptosis in transgenic mice expressing the P301L mutated form of human tau. Mol Med 14:309–317
Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K et al (2005) Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci 25:10637–10647
Richards BA, Chertkow H, Singh V, Robillard A, Massoud F, Evans AC et al (2009) Patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging 30:1626–1636
Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP et al (2004) Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest 114:121–130
Rocher AB, Crimins JL, Amatrudo JM, Kinson MS, Todd-Brown MA, Lewis J et al (2009) Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs. Exp Neurol 223:385–393
Rohn TT, Head E (2008) Caspase activation in Alzheimer’s disease: early to rise and late to bed. Rev Neurosci 19:383–393
Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M et al (2005) Tau suppression in a neurodegenerative mouse model improves memory function. Science 309:476–481
Schindowski K, Bretteville A, Leroy K, Begard S, Brion JP, Hamdane M et al (2006) Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol 169:599–616
Spires TL, Orne JD, SantaCruz K, Pitstick R, Carlson GA, Ashe KH et al (2006) Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol 168:1598–1607
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 72:1858–1862
Acknowledgments
We thank Claire Folger for her generous assistance and consultation on laboratory procedures over the course of this study. Grant Support: NIH/NIA R01 AG025062 (J. Luebke) and # P01 AG00001 (J. Luebke; A. Peters); NIH/NINDS R01 NS046355 and Alzheimer’s Association IIRG-06-27277 (J. Lewis).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ludvigson, A.E., Luebke, J.I., Lewis, J. et al. Structural abnormalities in the cortex of the rTg4510 mouse model of tauopathy: a light and electron microscopy study. Brain Struct Funct 216, 31–42 (2011). https://doi.org/10.1007/s00429-010-0295-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-010-0295-4