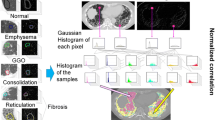
Abstract
Quantitative computed tomography (QCT) has recently gained an important role in the functional assessment of chronic lung disease. Its capacity in diagnostic, staging, and prognostic evaluation in this setting is similar to that of traditional pulmonary function testing. Furthermore, it can demonstrate lung injury before the alteration of pulmonary function test parameters, and it enables the classification of disease phenotypes, contributing to the customization of therapy and performance of comparative studies without the intra- and inter-observer variation that occurs with qualitative analysis. In this review, we address technical issues with QCT analysis and demonstrate the ability of this modality to answer clinical questions encountered in daily practice in the management of patients with chronic lung disease.





Similar content being viewed by others
Abbreviations
- CALIPER:
-
Computer-Aided Lung Informatics for Pathology Evaluation and Rating
- COPD:
-
Chronic obstructive pulmonary disease
- CT:
-
Computed tomography
- DTA:
-
Data-driven textural analysis
- FEV1 :
-
Forced expiratory volume in the first second
- FVC:
-
Forced vital capacity
- HAAs:
-
High-attenuation areas
- HU:
-
Hounsfield units
- ILD:
-
Interstitial lung disease
- MESA:
-
Multi-Ethnic Study of Atherosclerosis
- NLI:
-
Normal lung index
- PFT:
-
Pulmonary function test
- PRM:
-
Parametric response mapping
- QCT:
-
Quantitative computed tomography
- SPIROMICS:
-
Sub-Populations and Intermediate Outcome Measures in COPD Study
References
Chen A, Karwoski RA, Gierada DS et al (2020) Quantitative CT analysis of diffuse lung disease. Radiographics 40:28–43. https://doi.org/10.1148/rg.2020190099
Matsuoka S, Yamashiro T, Washko GR et al (2010) Quantitative CT assessment of chronic obstructive pulmonary disease. Radiographics 30:55–66. https://doi.org/10.1148/rg.301095110
Mascalchi M, Camiciottoli G, Diciotti S (2017) Lung densitometry: why, how and when. J Thorac Dis 9:3319–3345. https://doi.org/10.21037/jtd.2017.08.17
Cheng T, Li Y, Pang S et al (2019) Normal lung attenuation distribution and lung volume on computed tomography in a Chinese population. Int J Chron Obstruct Pulmon Dis 14:1657–1668. https://doi.org/10.2147/COPD.S187596
Barros MC, Hochhegger B, Altmayer S et al (2021) The normal lung index from quantitative computed tomography for the evaluation of obstructive and restrictive lung disease. J Thorac Imaging Publish Ah. https://doi.org/10.1097/RTI.0000000000000629
Shin KE, Chung MJ, Jung MP et al (2011) Quantitative computed tomographic indexes in diffuse interstitial lung disease. J Comput Assist Tomogr 35:266–271. https://doi.org/10.1097/RCT.0b013e31820ccf18
Humphries SM, Yagihashi K, Huckleberry J et al (2017) Idiopathic pulmonary fibrosis: data-driven textural analysis of extent of fibrosis at baseline and 15-month follow-up. Radiology 285:270–278. https://doi.org/10.1148/radiol.2017161177
Bartholmai BJ, Raghunath S, Karwoski RA et al (2013) Quantitative computed tomography imaging of interstitial lung diseases. J Thorac Imaging 28:298–307. https://doi.org/10.1097/RTI.0b013e3182a21969
Maldonado F, Moua T, Rajagopalan S et al (2014) Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur Respir J 43:204–212. https://doi.org/10.1183/09031936.00071812
Jacob J, Bartholmai BJ, Rajagopalan S et al (2017) Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J 49:1601011. https://doi.org/10.1183/13993003.01011-2016
Galbán CJ, Han MK, Boes JL et al (2012) Computed tomography–based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med 18:1711–1715. https://doi.org/10.1038/nm.2971
Sieren JP, Newell JD, Barr RG et al (2016) SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med 194:794–806. https://doi.org/10.1164/rccm.201506-1208PP
Hara AK, Paden RG, Silva AC et al (2009) Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. Am J Roentgenol 193:764–771. https://doi.org/10.2214/AJR.09.2397
Hammond E, Sloan C, Newell JD et al (2017) Comparison of low- and ultralow-dose computed tomography protocols for quantitative lung and airway assessment. Med Phys 44:4747–4757. https://doi.org/10.1002/mp.12436
Wisselink HJ, Pelgrim GJ, Rook M et al (2021) Ultra-low-dose CT combined with noise reduction techniques for quantification of emphysema in COPD patients: an intra-individual comparison study with standard-dose CT. Eur J Radiol 138:109646. https://doi.org/10.1016/j.ejrad.2021.109646
Chen H, Zeng Q, Zhang M et al (2017) Quantitative low-dose computed tomography of the lung parenchyma and airways for the differentiation between chronic obstructive pulmonary disease and asthma patients. Respiration 94:366–374. https://doi.org/10.1159/000478531
Heussel CP, Kappes J, Hantusch R et al (2010) Contrast enhanced CT-scans are not comparable to non-enhanced scans in emphysema quantification. Eur J Radiol 74:473–478. https://doi.org/10.1016/j.ejrad.2009.03.023
Fain SB, Lynch DA, Hatt C (2020) Invited commentary on “quantitative CT analysis of diffuse lung disease.” Radiographics 40:E1–E3. https://doi.org/10.1148/rg.2020200005
Sousa C, Rodrigues M, Carvalho A et al (2019) Diffuse smoking-related lung diseases: insights from a radiologic-pathologic correlation. Insights Imaging 10:73. https://doi.org/10.1186/s13244-019-0765-z
Lynch DA, Austin JHM, Hogg JC et al (2015) CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society. Radiology 277:192–205. https://doi.org/10.1148/radiol.2015141579
Mohamed Hoesein FAA, de Hoop B, Zanen P et al (2011) CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax 66:782–787. https://doi.org/10.1136/thx.2010.145995
Schroeder JD, McKenzie AS, Zach JA et al (2013) Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. Am J Roentgenol 201:W460–W470. https://doi.org/10.2214/AJR.12.10102
Lynch DA, Al-Qaisi MA (2013) Quantitative computed tomography in chronic obstructive pulmonary disease. J Thorac Imaging 28:284–290. https://doi.org/10.1097/RTI.0b013e318298733c
Bakker JT, Klooster K, Vliegenthart R, Slebos D-J (2021) Measuring pulmonary function in COPD using quantitative chest computed tomography analysis. Eur Respir Rev 30:210031. https://doi.org/10.1183/16000617.0031-2021
Kirby M, Smith BM, Tanabe N et al (2021) Computed tomography total airway count predicts progression to COPD in at-risk smokers. ERJ Open Res 7:00307–02021. https://doi.org/10.1183/23120541.00307-2021
Mohammed N, Kestin LL, Grills IS et al (2011) Rapid disease progression with delay in treatment of non–small-cell lung cancer. Int J Radiat Oncol 79:466–472. https://doi.org/10.1016/j.ijrobp.2009.11.029
Pompe E, Galbán CJ, Ross BD et al (2017) Parametric response mapping on chest computed tomography associates with clinical and functional parameters in chronic obstructive pulmonary disease. Respir Med 123:48–55. https://doi.org/10.1016/j.rmed.2016.11.021
Regan EA, Lynch DA, Curran-Everett D et al (2015) Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med 175:1539. https://doi.org/10.1001/jamainternmed.2015.2735
Ash SY, San José Estépar R, Fain SB et al (2021) Relationship between emphysema progression at CT and mortality in ever-smokers: results from the COPDGene and ECLIPSE cohorts. Radiology 299:222–231. https://doi.org/10.1148/radiol.2021203531
Konietzke P, Wielpütz MO, Wagner WL et al (2020) Quantitative CT detects progression in COPD patients with severe emphysema in a 3-month interval. Eur Radiol 30:2502–2512. https://doi.org/10.1007/s00330-019-06577-y
Loeh B, Brylski LT, von der Beck D et al (2019) Lung CT densitometry in idiopathic pulmonary fibrosis for the prediction of natural course, severity, and mortality. Chest 155:972–981. https://doi.org/10.1016/j.chest.2019.01.019
Easthausen I, Podolanczuk A, Hoffman E et al (2020) Reference values for high attenuation areas on chest CT in a healthy, never-smoker, multi-ethnic sample: the MESA study. Respirology 25:855–862. https://doi.org/10.1111/resp.13783
Podolanczuk AJ, Oelsner EC, Barr RG et al (2016) High attenuation areas on chest computed tomography in community-dwelling adults: the MESA study. Eur Respir J 48:1442–1452. https://doi.org/10.1183/13993003.00129-2016
Lynch DA (2007) Quantitative CT of fibrotic interstitial lung disease. Chest 131:643–644. https://doi.org/10.1378/chest.06-2955
Jankharia B, Angirish B (2021) Computer-aided quantitative analysis in interstitial lung diseases—a pictorial review using CALIPER. Lung India 38:161. https://doi.org/10.4103/lungindia.lungindia_244_20
Silva M, Milanese G, Seletti V et al (2018) Pulmonary quantitative CT imaging in focal and diffuse disease: current research and clinical applications. Br J Radiol. https://doi.org/10.1259/bjr.20170644
Kim GHJ, Weigt SS, Belperio JA et al (2020) Prediction of idiopathic pulmonary fibrosis progression using early quantitative changes on CT imaging for a short term of clinical 18–24-month follow-ups. Eur Radiol 30:726–734. https://doi.org/10.1007/s00330-019-06402-6
Best AC, Meng J, Lynch AM et al (2008) Idiopathic pulmonary fibrosis: physiologic tests, quantitative CT indexes, and CT visual scores as predictors of mortality. Radiology 246:935–940. https://doi.org/10.1148/radiol.2463062200
De Giacomi F, Raghunath S, Karwoski R et al (2018) Short-term automated quantification of radiologic changes in the characterization of idiopathic pulmonary fibrosis versus nonspecific interstitial pneumonia and prediction of long-term survival. J Thorac Imaging 33:124–131. https://doi.org/10.1097/RTI.0000000000000317
Jacob J, Bartholmai BJ, Rajagopalan S et al (2017) Unclassifiable-interstitial lung disease: outcome prediction using CT and functional indices. Respir Med 130:43–51. https://doi.org/10.1016/j.rmed.2017.07.007
Haruna A, Muro S, Nakano Y et al (2010) CT scan findings of emphysema predict mortality in COPD. Chest 138:635–640. https://doi.org/10.1378/chest.09-2836
Barros MC, Hochhegger B, Altmayer S et al (2018) Quantitative computed tomography phenotypes, spirometric parameters, and episodes of exacerbation in heavy smokers: an analysis from South America. PLoS ONE 13:e0205273. https://doi.org/10.1371/journal.pone.0205273
Johannessen A, Skorge TD, Bottai M et al (2013) Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med 187:602–608. https://doi.org/10.1164/rccm.201209-1722OC
Wu X, Kim GH, Salisbury ML et al (2019) Computed tomographic biomarkers in idiopathic pulmonary fibrosis. The future of quantitative analysis. Am J Respir Crit Care Med 199:12–21. https://doi.org/10.1164/rccm.201803-0444PP
Hochhegger B, Irion KL, Marchiori E, Moreira JS (2011) Reconstruction algorithms influence the follow-up variability in the longitudinal CT emphysema index measurements. Korean J Radiol 12:169. https://doi.org/10.3348/kjr.2011.12.2.169
Ohno Y, Aoyagi K, Takenaka D et al (2021) Machine learning for lung CT texture analysis: improvement of inter-observer agreement for radiological finding classification in patients with pulmonary diseases. Eur J Radiol 134:109410. https://doi.org/10.1016/j.ejrad.2020.109410
Acknowledgements
We thank Imbio (Minneapolis, MN) for providing us with a sample report of their software for this review.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
M.C.B. and S.A. wrote the first draft. A.R.C., R.R., and M.Z. contributed to the writing of the manuscript draft and literature review. T.L.M., P.P., A.M, B.M., M.C. helped with the preparation of figures and provided intellectual input. B.H. conceptualized the manuscript and supervised its writing. All authors reviewed the final version and contributed to the editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barros, M.C., Altmayer, S., Carvalho, A.R. et al. Quantitative Computed Tomography: What Clinical Questions Can it Answer in Chronic Lung Disease?. Lung 200, 447–455 (2022). https://doi.org/10.1007/s00408-022-00550-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-022-00550-1